Download Files:
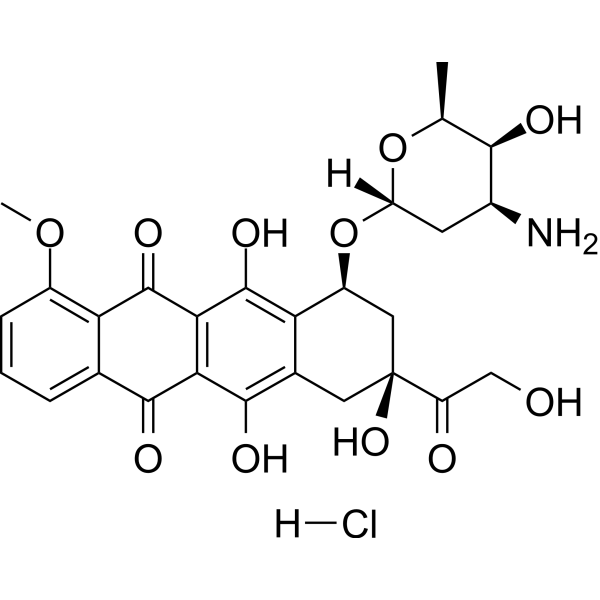
Doxorubicin (hydrochloride)
$60 – $474
Products Details
Product Description
– Doxorubicin (Hydroxydaunorubicin) hydrochloride, a cytotoxic anthracycline antibiotic, is an anti-cancer chemotherapy agent. Doxorubicin hydrochloride is a potent human DNA topoisomerase I and topoisomerase II inhibitor with IC50s of 0.8 μM and 2.67 μM, respectively. Doxorubicin hydrochloride reduces basal phosphorylation of AMPK and its downstream target acetyl-CoA carboxylase. Doxorubicin hydrochloride induces apoptosis and autophagy[1][2][3].
Web ID
– HY-15142
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture and light)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C27H30ClNO11
Citations
– ACS Appl Mater Interfaces. 2020 Oct 21;12(42):47330-47341.|ACS Appl Mater Interfaces. 2022 Aug 24.|ACS Nano. 2020 Nov 24;14(11):15904-15918.|Acta Pharmacol Sin. 2023 May 24.|Adv Funct Mater. 10 October 2021.|Adv Funct Mater. 23 October 2021.|Adv Healthc Mater. 2020 Nov;9(22):e2001633.|Adv Healthc Mater. 2021 Sep 8;e2101222.|Adv Sci (Weinh). 2020 Sep 28;7(21):2001364.|Advanced Therapeutics. 2021 Apr 14.|Am J Cancer Res. 2021 Apr 15;11(4):1428-1445.|Am J Transl Res. 2021 Sep 15;13(9):10112-10126.|Angiogenesis. 2021 Jan 17.|Ann Transl Med. 2020 Dec;8(23):1570.|Anticancer Drugs. 2022 Mar 1;33(3):308-319.|Arab J Chem. July 2022, 103934.|Autophagy. 2021 Dec 10;1-19.|Autophagy. 2021 Mar 22;1-17.|Biochem Biophys Res Commun. 2019 Dec 10;520(3):544-550.|Biochem Biophys Res Commun. 2020 Dec 10;533(3):304-312.|Bioengineered. 2022 Apr;13(4):9467-9481.|Biol Direct. 2023 Oct 9;18(1):63.|Biomacromolecules. 2022 Sep 14.|Biomater Adv. 17 October 2022, 213161.|Biomed Pharmacother. 2020 Oct;130:110534.|Biomed Pharmacother. 2021 May 29;140:111779.|Biomed Pharmacother. 2021 May;137:111378.|Biomed Pharmacother. 2021, 111308.|Biomed Pharmacother. 2021, 111531.|bioRxiv. 2020 Jun.|bioRxiv. 2023 Feb 7.|bioRxiv. 2023 Jan 13.|bioRxiv. 2023 May 12.|bioRxiv. 2023 Sep 16.|Breast Cancer Res Treat. 2021 Jul 8.|Cancer Biol Med. 2021 Feb 15; 18(1): 184-198.|Cancer Biol Med. 2021 Feb.|Cancer Lett. 2021 Aug 10;S0304-3835(21)00395-5.|Cancer Lett. 2022 Jun 28;536:215651.|Cancer Sci. 2022 Jun;113(6):2008-2021.|Cancers (Basel). 2021, 13(13), 3323.|Cancers (Basel). 2022, 14(3), 683.|Cell Death Differ. 2022 Jan 15.|Cell Death Dis. 2020 Sep 15;11(9):756.|Cell Death Dis. 2022 Aug 24;13(8):731.|Cell Death Discov. 2021 Aug 5;7(1):204.|Cell Metab. 2022 Feb 7;34(3):424-440.e7.|Cell Rep. 2021 Aug 24;36(8):109568.|Cell Rep. 2023 Mar 20;42(3):112275.|Cell Rep. 2023, 42(1): 111916.|Cell Stem Cell. 2021 Sep 14;S1934-5909(21)00343-X.|Cells. 2022 Dec 29;12(1):145.|Cells. 2022, 11(17), 2642.|Cells. 2023, 12(1), 145.|Chem Biodivers. 2022 Sep 1.|Chem Eng J. 2020, 127874.|China Biotechnology. 2017, 37(4): 68-75.|Circ Heart Fail. 2021 Oct;14(10):e008220.|Colloid Interface Sci Commun. 2019 Jan;28:69-74. |Colloid Surface B. 2021, 111839.|Colloids Surf B Biointerfaces. 2018 Jul 10;171:176-185.|Colloids Surf B Biointerfaces. 2019 Jan 1;173:27-35.|Department of Pharmaceutical Sciences, University of Toronto. 2019 Oct.|Dev Cell. 2018 Sep 24;46(6):681-695.e5.|DNA Repair . 13 March 2022, 103319.|Drug Deliv Transl Res. 2023 Mar 27.|EBioMedicine. 2021 Jul;69:103456.|Elife. 2022 May 3;11:e69255.|Eur Heart J Open. 2023 Oct 9.|Eur J Pharm Biopharm. 2018 Aug;129:88-103.|Expert Opin Drug Deliv. 2021 Mar 11.|FEBS Open Bio. 2021 Nov 14.|Free Radic Biol Med. 2019 Jan;130:557-567.|Free Radic Biol Med. 2021 Feb 1;163:141-152.|Free Radic Biol Med. 2023 Apr 24;S0891-5849(23)00378-7.|Free Radical Bio Med. 2021 Jan 6.|Front Cell Dev Biol. 2021 Dec 24;9:781792.|Front Endocrinol (Lausanne). 2018 Mar 22;9:120. |Harvard Medical School LINCS LIBRARY|Heliyon. 2023 Jul 13.|Hepatology. 2020 May;71(5):1660-1677. |Hereditas. 2023 Oct 31;160(1):36.|Inorganica Chimica Acta. 468 (2017) 270-279.|Int Immunopharmacol. 2021 Oct 26;101(Pt A):108264.|Int J Biol Sci. 2021 Jul 25;17(12):3255-3267.|Int J Med Sci. 2021 Jan 1;18(2):325-334.|Int J Mol Sci. 2019 Mar 5;20(5). pii: E1125. |Int J Mol Sci. 2022 Nov 19;23(22):14385.|Int J Oncol. 2023 Feb 8.|Int J Pharm. 2022 May 25;620:121761.|Int J Pharm. 2023 Feb 24;122779.|Int J Radiat Oncol Biol Phys. 2022 Jul 30;S0360-3016(22)02596-2.|J Biochem Mol Toxicol. 2022 Apr 25:e23077.|J Biomater Sci Polym Ed. 2018 Aug;29(12):1482-1497. |J Biomater Sci Polym Ed. 2021 Mar 11;1-13.|J Bone Oncol. October 2021, 100391.|J Cardiovasc Transl Res. 2020 Jul 16. |J Cell Biol. 2023 Jan 2;222(1):e202202110.|J Cell Physiol. 2021 Apr 26.|J Control Release. 2019 Jun 26;307:247-260. |J Control Release. 2022 Feb 21;S0168-3659(22)00100-6.|J Ethnopharmacol. 2022 Aug 31;115676.|J Extracell Vesicles. 2022 Aug;11(8):e12255.|J Hematol Oncol. 2021 Oct 29;14(1):178.|J Immunother Cancer. 2022 Aug;10(8):e004006.|J Med Chem. 2023 Jul 6.|J Mol Med (Berl). 2019 Aug;97(8):1183-1193.|J Nanobiotechnology. 2023 Feb 10;21(1):50.|J Org Chem. 2021 Sep 16.|J Transl Med. 2021 Mar 21;19(1):117.|J Virol. 2021 May 26;JVI0076021.|Journal of New Chinese Medicine. 2016(7):278-282.|Lett Appl Microbiol. 2022 Feb 25.|Mater Chem Phys. 2021, 124596.|Mater Today Bio. 2023 Oct, 22, 100780.|Materials Today Chemistry. March 2022, 100637.|MedComm. 2021 Jun 3.|Mol Carcinog. 2021 Apr 18.|Mol Cell Biochem. 2021 Feb;476(2):1233-1243.|Mol Med Rep. 2020 Aug;22(2):915-925.|Mol Ther. 2019 May 8;27(5):1051-1065. |Nanoscale Res Lett. 2018 Nov 3;13(1):350. |Nanotheranostics. 2021 Jan 1;5(2):143-154.|Nanotheranostics. 2021; 5(2): 143-154.|Nat Cell Biol. 2020 Sep;22(9):1056-1063.|Nat Commun. 2018 Oct 8;9(1):4139. |Nat Commun. 2023 Apr 13;14(1):2109.|Nat Med. 2016 May;22(5):547-56. |Nat Protoc. 2021 Jan;16(1):405-430.|Nature. 2023 Jun;618(7964):374-382.|NPJ Breast Cancer. 2022 May 6;8(1):60.|Oncogene. 2022 Jun 30.|Oncotarget. 2017 Nov 24;8(67):111190-111212. |Oxid Med Cell Longev. 2022 Apr 5;2022:4740931.|Oxid Med Cell Longev. 2023 Jan 14;2023:9966355.|Patent. US20180185310A1.|Patent. US20180185346A1.|Patent. US20180185379A1.|Patent. US20180185386A1.|Patent. US20180185387A1.|Patent. US20180185388A1|Patent. US20180185389A1.|Patent. US20180193355A1.|Patent. US20200085841A1 |Patent. US20200345752A1.|Patent. US20210275477A1.|Patent. US20220016148A1.|Patent. US20230190773A1.|Pathol Res Pract. January 2022, 153735.|Pharmacol Res. 2021 Apr 30;169:105642.|Phytochemistry. 2021 Jul 31;190:112892.|Phytomedicine. 2022 Jan 29;99:153964.|Phytomedicine. 2023 Jun 10, 154922.|Phytomedicine. 2023 Mar 10.|PLoS Pathog. 2020 Mar 24;16(3):e1008429.|Polym Bull (Berl). 30 September 2022.|Redox Biol. 2021 Aug 31;46:102120.|Research Square Preprint. 2020 Jul.|Research Square Preprint. 2021 Nov.|Research Square Preprint. 2021 Sep.|Research Square Preprint. 2023 Nov 25.|Research Square Preprint. 2023 Sep 20.|Sci Adv. 2023 Mar 10;9(10):eadd8539.|Shock. 2023 Jul 1;60(1):100-109.|Signal Transduct Target Ther. 2023 Feb 3;8(1):51.|Small. 2023 Feb 7;e2205606.|SSRN. 2023 Aug 14.|SSRN. 2023 Nov 28.|Ther Deliv. 2017 Mar;8(5):249-264.|Theranostics. 2020 May 17;10(15):6581-6598. |Thyroid. 2019 Jun;29(6):809-823. |TMR Modern Herbal Medicine. 2021 Jul.|Transl Androl Urol. Jul 29, 2022.|Transl Oncol. 2021 Nov 22;15(1):101272.|Ulsan National Institute of Science and Technology. 2023 Aug.|Universiti Tunku Abdul Rahman. 2023 Sep 11.|University of Szeged. Department of Biochemistry and Molecular Biology Faculty of Science and Informatics. 2020 Nov.|ACS Omega. 2019 Jul 11;4(7):12036-12042.|Acta Biomater. 2020 Jun;109:229-243.|Adv Healthc Mater. 2019 Sep;8(18):e1900543.|Adv Sci (Weinh). 2022 May;9(15):e2105894.|Adv Sci (Weinh). 2022 Oct;9(30):e2201210.|Adv Sci (Weinh). 2023 Mar 26;e2206007.|Am J Physiol Cell Physiol. 2023 Nov 27.|Anal Chem. 2022 Sep 19.|Antioxid Redox Signal. 2023 Jun 2.|Artif Cells Nanomed Biotechnol. 2023 Dec;51(1):120-130.|BBA-Mol Cell Res. 2022: 119409.|Biochem Biophys Res Commun. 2019 Sep 24;517(3):538-544.|Biochem Biophys Res Commun. 2020 Jan 15;521(3):596-602.|Biochem Biophys Res Commun. 2023 Nov 18, 149244.|Biochem Biophys Res Commun. 2023 Nov 24, 149314.|Biochem Pharmacol. 2020 Apr;174:113795.|Biochem Pharmacol. 2020 May;175:113888.|Biochem Pharmacol. 2023 Aug 25;115769.|Biochem Pharmacol. 2020 May;175:113856.|Biochim Biophys Acta Mol Cell Res. 2022: 119411.|Biofabrication. 2022 Apr 13;14(3).|Biomacromolecules. 2023 Jan 13.|Biomaterials. 2023 Oct, 301, 122236.|Biomed Pharmacother. 2020 Mar;123:109803.|Biomed Pharmacother. 2023 Apr 13;162:114691.|Biomed Pharmacother. 2023 Apr 21;162:114733.|Biomed Pharmacother. 2023 Jan;157:114087.|Biomed Pharmacother. 2023 Oct 6:168:115654.|Biomolecules. 2022 Feb 12;12(2):299.|Bioorg Chem. 17 October 2022, 106206.|bioRxiv. 2023 Apr 14.|bioRxiv. 2023 Feb 27.|bioRxiv. 2023 Sep 5.|BMC Cancer. 2019 Jun 18;19(1):602. |Br J Pharmacol. 2022 Jan 18.|Breast Cancer Res Treat. 2023 May 19.|Breast Cancer Res. 2023 Oct 4;25(1):115.|Cancer Cell Int. 2019 Jul 12;19:179. |Cancer Lett. 2022 Nov 30;216028.|Cancers (Basel). 2022, 14(13), 3068.|Cancers (Basel). 2023 Feb 1;15(3):930.|Carcinogenesis. 2022 Sep 30;bgac078.|Cell Biochem Funct. 2022 Apr 29.|Cell Biosci. 2022 Jan 6;12(1):6.|Cell Chem Biol. 2020 Nov 19;27(11):1359-1370.e8.|Cell Death Differ. 2022 Oct;29(10):1982-1995.|Cell Death Dis. 2019 Nov 25;10(12):887. |Cell Death Dis. 2022 Apr 5;13(4):305.|Cell Death Dis. 2019 Sep 11;10(9):668.|Cell Mol Neurobiol. 2022 Oct 20.|Cell Physiol Biochem. 2017;42(3):965-973.|Cell Res. 2018 Dec;28(12):1171-1185. |Cell Signal. 2017 May 1;36:108-116. |Cell Signal. 2023 Mar 14;110655.|Cell Stress Chaperones. 2022 Dec 13.|Chem Eng J. 15 October 2022, 137110.|Chronic Diseases Prevention Review. 2019 Feb.|Clin Cancer Res. 2020 Apr 15;26(8):2011-2021. |Clin Exp Pharmacol Physiol. 2023 Aug 13.|Clin Transl Oncol. 2023 Feb 27.|Commun Biol. 2022 Nov 26;5(1):1295.|Eur J Med Chem. 2023 Nov 24, 115975.|Eur J Med Chem. 2023 Oct 5, 258, 115602.|Eur J Pharm Sci. 2023 May 21;106472.|Evid Based Complement Alternat Med. 2022 Sep 28;2022:7760945.|Exp Ther Med. 2023 May 16.|FASEB J. 2022 Sep;36(9):e22495.|FASEB J. 2023 Dec;37(12):e23284.|FASEB J. 2023 Jun;37(6):e22982.|Food Funct. 17th January 2022.|Food Funct. 2022 Aug 24.|Free Radic Biol Med. 2022 Oct 11;S0891-5849(22)00898-X.|Front Cell Dev Biol. 2021 May 31;9:661602.|Front Immunol. 2020 May 5;11:802.|Front Oncol. 2020 Mar 13;10:308.|Gels. 2022 Apr 12;8(4):237.|Gels. 2022, 8(9), 594.|IEEE Access. 2023 May 31.|Infect Immun. 2019 Dec 17;88(1):e00697-19.|Int Immunopharmacol. 2022 Nov 26;114:109450.|Int J Biol Sci. 2022 Apr 11;18(7):2882-2897.|Int J Biol Sci. 2022 Feb 7;18(4):1724-1736.|Int J Clin Exp Pathol. 2017;10(3):3033-3042.|Int J Mol Sci. 2023 Oct 20, 24(20), 15405.|Int J Mol Sci. 2023, 24(2), 1194.|Int J Nanomedicine. 2017 Mar 16;12:2081-2108.|Int J Oncol. 2022 Dec;61(6):148.|International Journal of Biology and Life Sciences. 2023 May 22.|iScience. 2023 Oct 17.|iScience. 6 September 2022, 105064.|iScience. 6 September 2022, 105081.|J Agric Food Chem. June 30, 2022.|J Cell Mol Med. 2023 Jun 29.|J Cell Mol Med. 2019 Sep;23(9):6034-6047.|J Clin Invest. 2018 Jan 2;128(1):483-499. |J Drug Target. 2020 Feb;28(2):186-194. |J Electroanal Chem. 2023 Sep 16, 117808.|J Environ Chem Eng. February 2022, 107078.|J Enzyme Inhib Med Chem. 2019 Dec;34(1):117-123. |J Ethnopharmacol. 2020 Jul 15;257:112789.|J Exp Clin Cancer Res. 2018 Sep 19;37(1):232. |J Exp Clin Cancer Res. 2019 Aug 14;38(1):353. |J Food Biochem. 2022 Jan 5;e14065.|J Immunol. 2022 Oct 24;ji2200182.|J Med Virol. 2019 Oct;91(10):1818-1829. |J Nanobiotechnology. 2023 Aug 26;21(1):297.|J Nat Prod. 2020 Feb 28;83(2):516-523.|J Photoch Photobio B. 2022: 112642.|J Toxicol Sci. 2023;48(8):469-479.|J Transl Med. 2023 Jan 9;21(1):9.|J Transl Med. 2023 Nov 17;21(1):823.|JCI Insight. 2022 Oct 18;e156485.|Life. 2022 Sep 2;12(9):1369.|Mar Drugs. 2022, 20(10), 591.|Mil Med Res. 2021 Dec 9;8(1):63.|Mol Biol Rep. 2022 Nov 9.|Mol Med Rep. 2020 Apr;21(4):1739-1748.|Mol Med Rep. 2020 Jul;22(1):67-76.|Mol Med Rep. 2023 Jan;27(1):17.|Mol Ther. 2023 Feb 18;S1525-0016(23)00079-5.|Molecules. 2020 Feb 14;25(4):836.|Nano Today. 2023 Aug, 51, 101898.|Nanomaterials. 2022, 12(24), 4478.|Nat Commun. 2019 Dec 2;10(1):5492. |Nucleic Acids Res. 2018 Apr 20;46(7):3284-3297.|Nutrients. 2022, 14(19), 4017.|Oncogenesis. 2023 Jun 24;12(1):34.|Oncol Lett. 2020 Jul;20(1):145-154.|Oncol Lett. 2023 Aug 30.|OPT Express. 1976-11986 (2021).|Org Chem Front. 11 Feb 2022.|Patent. US20230145200A1.|PeerJ. 2023 May 18.|Pharmaceutics. 2019 May 27;11(5):247. |Pharmaceutics. 2023 Oct 28, 15(11), 2550.|Pharmacogn Mag. 2023 Apr 14.|Pharmacol Res. 2022 Apr;178:106186.|Phytochemistry. 2022 Jul;199:113172.|Phytomedicine. 2022 Mar 2;99:154027.|Phytomedicine. 2023 Sep 7, 155074.|Phytother Res. 2023 Sep 1.|Plasma Process Polym. 2021 Feb 12.|PLoS One. 2023 Jul 27;18(7):e0288422.|Redox Biol. 18 December 2021, 102219.|Redox Biol. 2022 Jun;52:102310.|Research Square Preprint. 2021 Apr.|Research Square Preprint. 2021 Aug.|Research Square Preprint. 2022 Jun.|Research Square Preprint. 2023 Jun 16.|Research Square Print. November 29th, 2022.|RSC Adv. 2022, 12, 28104-28112.|Sci Rep. 2019 Oct 22;9(1):15099. |Sci Rep. 2019 Oct 23;9(1):15172. |Small Methods. 2022 Feb 2;e2101391.|SSRN. 2019 Nov.|Theranostics. 2019 Jan 24;9(3):761-777.|Theranostics. 2020 Jul 25;10(21):9477-9494. |Tissue Cell. 2023 Mar 17.|Toxicol Appl Pharmacol. 2022 Jul 29;116179.|Toxics. 2023 Nov 12, 11(11), 925.|Toxins. 2023 Mar 25.|Trop J Pharm Res. 2022; 21 (12): 2541-2548.
References
– [1]Nesstor Pilco-Ferreto, et al. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int J Oncol. 2016 Aug;49(2):753-62.|[2]Regine Lüpertz, et al. Dose- and time-dependent effects of doxorubicin on cytotoxicity, cell cycle and apoptotic cell death in human colon cancer cells. Toxicology. 2010 May 27;271(3):115-21.|[3]Penelope D Ottewell, et al. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008 Aug 20;100(16):1167-78.|[4]John L. Nitiss, et al. Targeting DNA topoisomerase II in cancer chemotherapy.Nat Rev Cancer. 2009 May;9(5):338-50.|[5]Hee-KyungRhee,et al. Synthesis, cytotoxicity, and DNA topoisomerase II inhibitory activity of benzofuroquinolinediones. Bioorg Med Chem. 2007 Feb 15;15(4):1651-8.|[6]P D Foglesong, et al. Doxorubicin inhibits human DNA topoisomerase I. Cancer Chemother Pharmacol. 1992;30(2):123-5.
CAS Number
– 25316-40-9
Molecular Weight
– 579.98
Compound Purity
– 99.60
SMILES
– COC1=C2C(C(C(C(O)=C(C[C@](C(CO)=O)(O)C[C@@H]3O[C@@]4([H])C[C@H](N)[C@H](O)[C@H](C)O4)C3=C5O)=C5C2=O)=O)=CC=C1.[H]Cl
Clinical Information
– Launched
Research Area
– Cancer; Infection
Solubility
– DMSO : 83.33 mg/mL (ultrasonic)|H2O : 50 mg/mL (ultrasonic;warming;heat to 60°C)
Target
– ADC Cytotoxin;AMPK;Antibiotic;Apoptosis;Autophagy;Bacterial;HBV;HIV;Mitophagy;Topoisomerase
Isoform
– Daunorubicins/Doxorubicins;HIV-1;Topo I;Topo II
Pathway
– Antibody-drug Conjugate/ADC Related;Anti-infection;Apoptosis;Autophagy;Cell Cycle/DNA Damage;Epigenetics;PI3K/Akt/mTOR
Product type
– Natural Products
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.