Download Files:
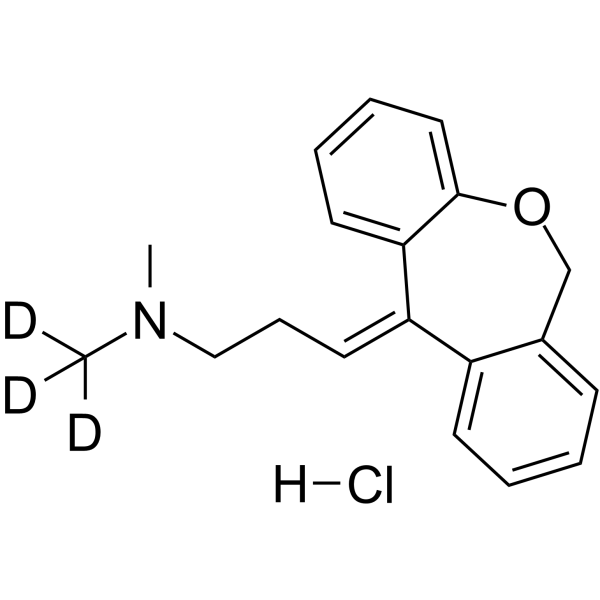
Doxepin-d3 (hydrochloride)
$150 – $640
Products Details
Product Description
– Doxepin-d3 (hydrochloride) is a deuterium labeled Doxepin Hydrochloride. Doxepin hydrochloride is an orally active tricyclic antidepressant. Doxepin hydrochloride is a potent and selective histamine receptor H1 antagonist. Doxepin hydrochloride is also a potent CYP450 inhibitor and significantly inhibits CYP450 2C19 and 1A2[1][2].
Web ID
– HY-B0725S
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Neuroscience-Neuromodulation
Molecular Formula
– C19H19D3ClNO
References
– [1]Hajak, G., et al., Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. J Clin Psychiatry, 2001. 62(6): p. 453-63.|[2]Gillman PK1. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007 Jul;151(6):737-48.
CAS Number
– 347840-07-7
Molecular Weight
– 318.86
Compound Purity
– 98.68
SMILES
– [2H]C(N(C)CC/C=C1C2=CC=CC=C2OCC3=CC=CC=C13)([2H])[2H].[H]Cl
Clinical Information
– No Development Reported
Research Area
– Neurological Disease
Solubility
– 10 mM in DMSO
Target
– Cytochrome P450;Histamine Receptor
Isoform
– H1 Receptor
Pathway
– GPCR/G Protein;Immunology/Inflammation;Metabolic Enzyme/Protease;Neuronal Signaling
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.