Download Files:
Desmethyl Erlotinib-d4
SKU
HY-13256AS-1 mg
Category Isotope-Labeled Compounds
Tags Cancer, Drug Metabolite;Isotope-Labeled Compounds, Metabolic Enzyme/Protease;Others
$1,120
Only 1000 item(s) left in stock.
Products Details
Product Description
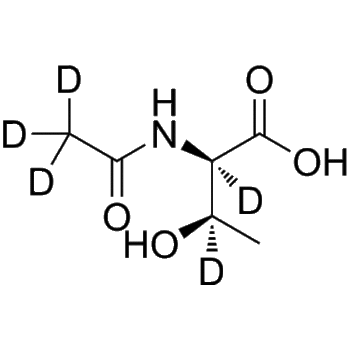
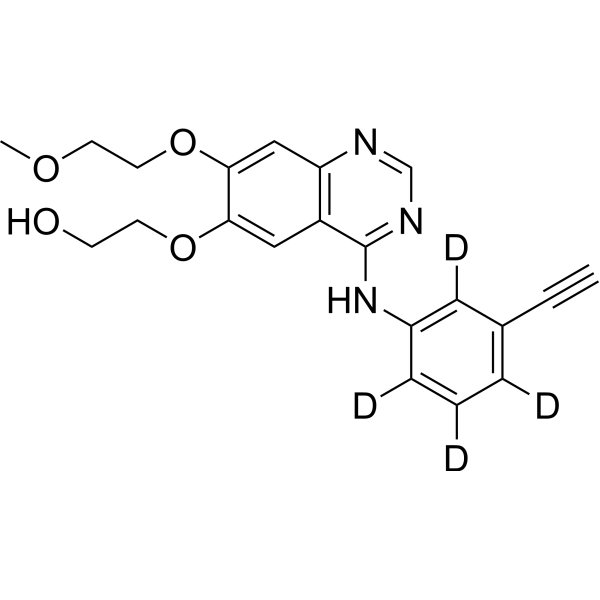
– Desmethyl Erlotinib-d4 is the deuterium labeled Desmethyl Erlotinib. Desmethyl Erlotinib (OSI-420 free base) is an active metabolite of Erlotinib. Erlotinib is a potent EGFR tyrosin kinase inhibitor[1][2]. Desmethyl Erlotinib-d4 is a click chemistry reagent, it contains an Alkyne group and can undergo copper-catalyzed azide-alkyne cycloaddition (CuAAc) with molecules containing Azide groups.
Web ID
– HY-13256AS
Shipping
– Room temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C21H17D4N3O4
References
– [1]Thappali SR, et al. Simultaneous Determination of Celecoxib, Erlotinib, and its Metabolite Desmethyl-Erlotinib (OSI-420) in Rat Plasma by Liquid chromatography/Tandem Mass Spectrometry with Positive/Negative Ion-Switching Electrospray Ionisation.Sci Pharm. 2012 Jul-Sep;80(3):633-46.|[2]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216.
CAS Number
– 1216420-11-9
Molecular Weight
– 383.43
SMILES
– N(C=1C2=C(C=C(OCCOC)C(OCCO)=C2)N=CN1)C3=C(C(C#C)=C(C(=C3[2H])[2H])[2H])[2H]
Clinical Information
– No Development Reported
Research Area
– Cancer
Solubility
– 10 mM in DMSO
Target
– Drug Metabolite;Isotope-Labeled Compounds
Pathway
– Metabolic Enzyme/Protease;Others
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.