Download Files:
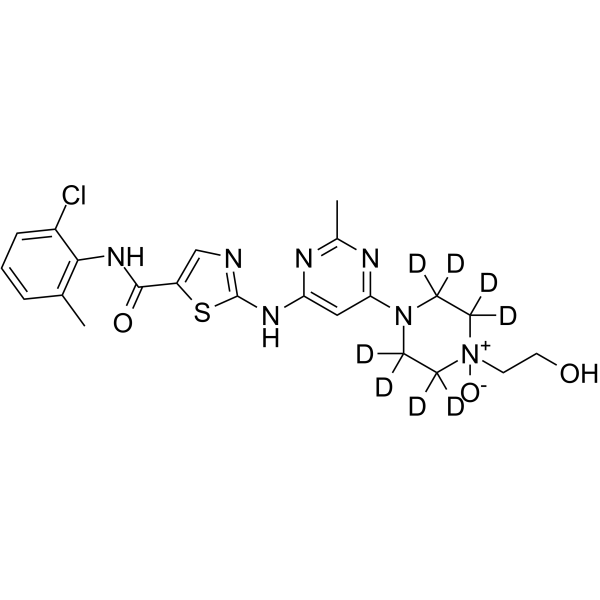
Dasatinib N-oxide-d8
SKU
HY-133794S-1 mg
Category Isotope-Labeled Compounds
Tags Cancer, Drug Metabolite;Isotope-Labeled Compounds, Metabolic Enzyme/Protease;Others
Products Details
Product Description
– Dasatinib N-oxide-d8 is the deuterium labeled Dasatinib N-oxide. Dasatinib N-oxide is a minor metabolite of Dasatinib. Dasatinib is a potent and orally active dual Src/Bcr-Abl inhibitor[1][2].
Web ID
– HY-133794S
Shipping
– Room temperature
Molecular Formula
– C22H18D8ClN7O3S
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216. |[2]Christopher LJ, et, al. Metabolism and disposition of dasatinib after oral administration to humans. Drug Metab Dispos. 2008 Jul;36(7):1357-64.|[3]Furlong MT, et, al. A validated LC-MS/MS assay for the simultaneous determination of the anti-leukemic agent dasatinib and two pharmacologically active metabolites in human plasma: application to a clinical pharmacokinetic study. J Pharm Biomed Anal. 2012 Jan 25;58:130-5.
CAS Number
– 1189988-36-0
Molecular Weight
– 512.05
SMILES
– OCC[N+]1([O-])C([2H])([2H])C([2H])([2H])N(C2=NC(C)=NC(NC3=NC=C(C(NC4=C(C)C=CC=C4Cl)=O)S3)=C2)C([2H])([2H])C1([2H])[2H]
Clinical Information
– No Development Reported
Research Area
– Cancer
Solubility
– 10 mM in DMSO
Target
– Drug Metabolite;Isotope-Labeled Compounds
Pathway
– Metabolic Enzyme/Protease;Others
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.