Download Files:
Dabigatran etexilate (mesylate)
SKU
HY-10274A-1 g
Category Reference compound
Tags Cardiovascular Disease, Metabolic Enzyme/Protease, Thrombin
$50 – $432
Products Details
Product Description
– Dabigatran etexilate mesylate (BIBR 1048MS) is an orally active proagent of Dabigatran (a direct inhibitor of thrombin). Dabigatran etexilate mesylate has anticoagulant effects and is used for the prophylaxis of venousthromboembolism and stroke due to atrial fibrillation[1].
Web ID
– HY-10274A
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
– C35H45N7O8S
Citations
– J Chromatogr Sci. 2016 Jul 29;54(9):1648-1651.|Biochem Pharmacol. 2016 Nov 1;119:76-84.
References
– [1]Blair HA, Keating GM. Dabigatran Etexilate: A Review in Nonvalvular Atrial Fibrillation. Drugs. 2017;77(3):331-344. |[2]Wienen W, et al. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally activeprodrug, dabigatran etexilate. Thromb Haemost. 2007 Jul;98(1):155-62.
CAS Number
– 872728-81-9
Molecular Weight
– 723.84
Compound Purity
– 99.93
SMILES
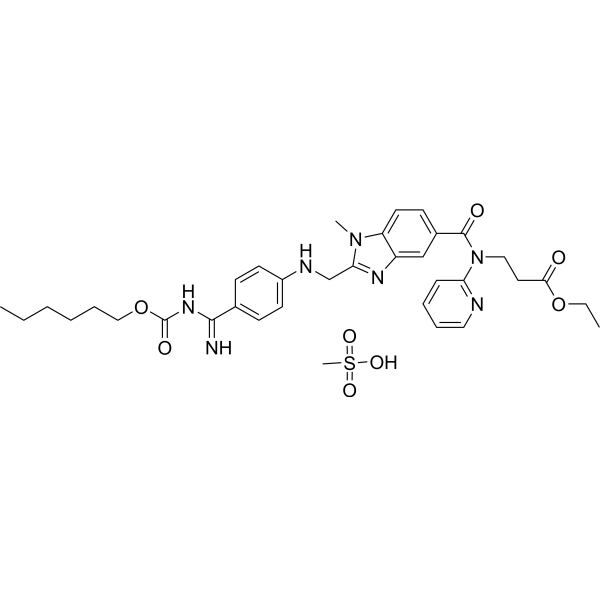
– O=C(N(C1=NC=CC=C1)CCC(OCC)=O)C2=CC=C3C(N=C(N3C)CNC4=CC=C(C(NC(OCCCCCC)=O)=N)C=C4)=C2.CS(=O)(O)=O
Clinical Information
– Launched
Research Area
– Cardiovascular Disease
Solubility
– DMSO : 25 mg/mL (ultrasonic)
Target
– Thrombin
Pathway
– Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.





