Download Files:
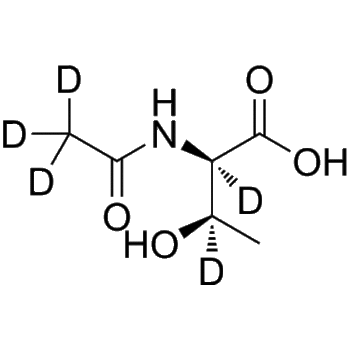
Dabigatran-13C,d3
SKU
HY-10163S3-1mg
Category Isotope-Labeled Compounds
Tags Cardiovascular Disease, Isotope-Labeled Compounds;Thrombin, Metabolic Enzyme/Protease;Others
Products Details
Product Description
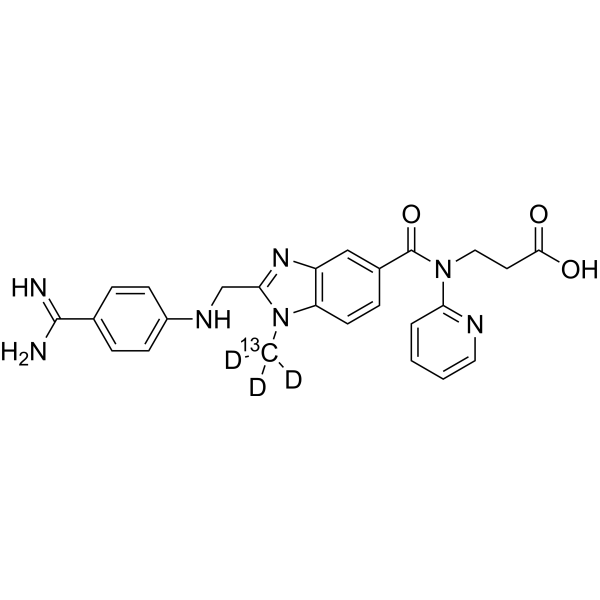
– Dabigatran-13C,d3 is the 13C- and deuterium labeled Dabigatran. Dabigatran (BIBR 953), an oral anticoagulant, is a reversible, potent, competitive direct thrombin inhibitor (Ki=4.5 nM). Dabigatran (BIBR 953) also inhibits thrombin-induced platelet aggregation (IC50=10 nM)[1][2].
Web ID
– HY-10163S3
Shipping
– Room temperature
Applications
– Neuroscience-Neuromodulation
Molecular Formula
– C24 13CH22D3N7O3
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-223.|[2]Hauel NH, et al. Structure-based design of novel potent nonpeptide thrombin inhibitors. J Med Chem. 2002 Apr 25;45(9):1757-66.|[3]Wienen W, et al. Effects of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate, on thrombus formation and bleeding time in rats. Thromb Haemost. 2007 Aug;98(2):333-8.|[4]Wienen W, Stassen JM, Priepke H, In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally activeprodrug, dabigatran etexilate. Thromb Haemost. 2007 Jul;98(1):155-62.
Molecular Weight
– 475.52
SMILES
– O=C(CCN(C1=NC=CC=C1)C(C2=CC(N=C(CNC3=CC=C(C(N)=N)C=C3)N4[13C]([2H])([2H])[2H])=C4C=C2)=O)O
Clinical Information
– No Development Reported
Research Area
– Cardiovascular Disease
Solubility
– 10 mM in DMSO
Target
– Isotope-Labeled Compounds;Thrombin
Pathway
– Metabolic Enzyme/Protease;Others
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.