Download Files:
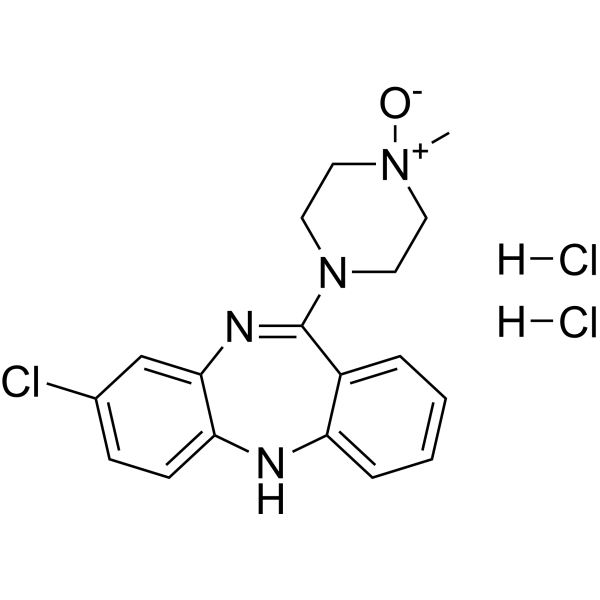
Clozapine N-oxide (dihydrochloride)
SKU
HY-17366A-10 mg
Category Reference compound
Tags Dopamine Receptor;Drug Metabolite;mAChR, GPCR/G Protein;Metabolic Enzyme/Protease;Neuronal Signaling, Neurological Disease
$58 – $95
Products Details
Product Description
– Clozapine N-oxide dihydrochloride is a major metabolite of Clozapine and a human muscarinic designer receptors (DREADDs) agonist. Clozapine N-oxide dihydrochloride activates the DREADD receptor hM3Dq and hM4Di. Clozapine N-oxide dihydrochloride can cross the blood-brain barrier[1][2][3][4]. Clozapine is a potent dopamine antagonist and also a potent and selective muscarinic M4 receptor (EC50=11 nM) agonist[5][6].
Web ID
– HY-17366A
Storage Temperature
– 4°C (Powder, stored under nitrogen)
Shipping
– Room Temperature
Applications
– Neuroscience-Neuromodulation
Molecular Formula
– C18H21Cl3N4O
Citations
– Cells. 2022 Dec 21;12(1):26.|Cells. 2023, 12(1), 26.|iScience. 2022: 105840.|Pharmacol Res. 2023 Apr 15;106773.|Acta Pharmacol Sin. 2023 Jan 25.|Acta Physiol. 2023 Jan 4;e13917.|Adv Sci (Weinh). 2023 Apr 14;e2204824.|Adv Sci (Weinh). 2023 Jun 16;e2301110.|Anesthesiology. 2021 Jul 13.|Anesthesiology. 2021 Jul 16.|Biol Psychiatry. 2017 May 1;81(9):737-747.|bioRxiv. 2023 Oct 9.|Brain Behav Immun. 2023 Jun 5;S0889-1591(23)00141-1.|Brain Commun. 30 August 2022.|Cell Metab. 2022 Feb 1;34(2):285-298.e7.|Cell Rep. 2021 Mar 23;34(12):108889.|Cell Rep. 2023 Dec 3;42(12):113551.|Cell Rep. 2023 Mar 21;42(4):112290.|CNS Neurosci Ther. 2022 Nov 15.|Commun Biol. 2021 Sep 16;4(1):1088.|Commun Biol. 2023 Jan 14;6(1):50.|Ecotoxicol Environ Saf. 2023 Jun 29;262:115205.|Elife. 2021 Sep 16;10:e67535.|FASEB J. 2020 Sep;34(9):11913-11924.|Front Cell Neurosci. 2021 Apr 30;15:671473.|Front Neurosci. 2020 Jul 14;14:646.|iScience. 2023 Aug 14.|iScience. 2023 Oct 20.|J Neuroinflammation. 2022 Jun 27;19(1):166.|J Neuroinflammation. 2022 May 27;19(1):123.|J Neuropathol Exp Neurol. 2023 Jul 21;nlad056.|J Neurosci. 2021 Oct 11;JN-RM-0881-21.|Life Metabolism. 2023 Feb 4.|Life Sci. 2020 Oct 1;258:118099.|Nat Commun. 2020 Jun 5;11(1):2847. |Nat Commun. 2020 Nov 27;11(1):6045.|Nat Commun. 2022 Apr 25;13(1):2233.|Nat Commun. 2023 Apr 17;14(1):2182.|Nat Neurosci. 2022 Nov 17.|Nat Neurosci. 2023 Apr;26(4):542-554.|Neural Regen Res. 2024 Feb;19(2):425-433.|Neurobiol Dis. 2023 May 12;106155.|Neurobiol Stress. 22 September 2022, 100492.|Neuropharmacology. 2018 Jun;135:474-486.|Neuropsychopharmacology. 2022 Dec 16.|Neurosci Bull. 2021 Jun 18.|Neurosci Bull. 2021 May;37(5):597-610.|Neurosci Bull. 2022 Nov 7.|Neurosci Bull. 2023 Jan 11.|Neuroscience. 2019 Aug 21;414:299-310. |P Natl Acad Sci USA. 13, 2021 118 (28) e2103505118.|Proc Natl Acad Sci U S A. 2019 Aug 13;116(33):16583-16592.|Proc Natl Acad Sci U S A. 2023 May 9;120(19):e2215590120.|Prog Neuropsychopharmacol Biol Psychiatry. 18 December 2021, 110496.|Psychoneuroendocrinology. 2020 Jul;117:104690.|Research Square Preprint. 2021 Mar.|Research Square Preprint. 2023 Sep 22.|Research Square Print. September 6th, 2022|SSRN. 2023 Jul 18.|Theranostics. 2023 May 11;13(9):2946-2961.|Transl Psychiatry. 2020 Jun 20;10(1):202.|University of Michigan, Horace H. Rackham School of Graduate Studies. 2018 Oct.
References
– [1]Joseph Cichon, et al. Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature. 2015 Apr 9;520(7546):180-5.|[2]Wess J, et al. Novel designer receptors to probe GPCR signaling and physiology. Trends Pharmacol Sci. 2013 Jul;34(7):385-92.|[3]Manvich DF, et al. The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci Rep. 2018 Mar 1;8(1):3840.|[4]van der Peet PL, et al. Gram scale preparation of clozapine N-oxide (CNO), a synthetic small molecule actuator for muscarinic acetylcholine DREADDs. MethodsX. 2018 Mar 23;5:257-267.|[5]Silva RR, et al. Evaluation of Functional Selectivity of Haloperidol, Clozapine, and LASSBio-579, an Experimental Compound With Antipsychotic-Like Actions in Rodents, at G Protein and Arrestin Signaling Downstream of the Dopamine D2 Receptor. Front Pharmacol. 2019 Jun 4;10:628.|[6]Zorn SH, et al. Clozapine is a potent and selective muscarinic M4 receptor agonist. Eur J Pharmacol. 1994 Nov 15;269(3):R1-2.
CAS Number
– 2250025-93-3
Molecular Weight
– 415.74
Compound Purity
– 99.97
SMILES
– C[N+]1([O-])CCN(C2=NC3=CC(Cl)=CC=C3NC4=CC=CC=C42)CC1.[H]Cl.[H]Cl
Clinical Information
– No Development Reported
Research Area
– Neurological Disease
Solubility
– DMSO : 240 mg/mL (ultrasonic)|H2O : 100 mg/mL (ultrasonic)
Target
– Dopamine Receptor;Drug Metabolite;mAChR
Isoform
– mAChR3;mAChR4
Pathway
– GPCR/G Protein;Metabolic Enzyme/Protease;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.







