Download Files:
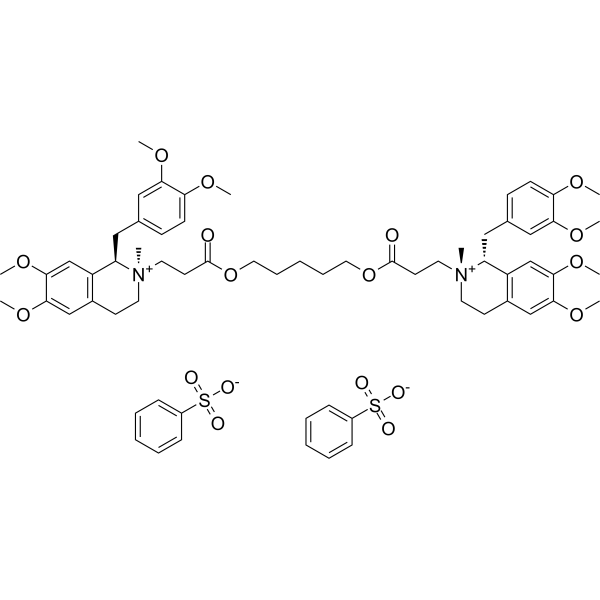
Cisatracurium (besylate)
SKU
HY-13596-100 mg
Category Reference compound
Tags Autophagy;Membrane Transporter/Ion Channel;Neuronal Signaling, Autophagy;nAChR, Neurological Disease; Cancer
$80 – $230
Products Details
Product Description
– Cisatracurium besylate (51W89) is a nondepolarizing neuromuscular blocking agent, antagonizing the action of acetylcholine by inhibiting neuromuscular transmission.
Web ID
– HY-13596
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture and light)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C65H82N2O18S2
References
– [1]Dear, G.J., et al., Identification of urinary and biliary conjugated metabolites of the neuromuscular blocker 51W89 by liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom, 1995. 9(14): p. 1457-64.|[2]Serra, C.S. and A.C. Oliveira, Cisatracurium: myographical and electrophysiological studies in the isolated rat muscle. Fundam Clin Pharmacol, 2006. 20(3): p. 291-8.
CAS Number
– 96946-42-8
Molecular Weight
– 1243.48
Compound Purity
– 98.0
SMILES
– O=C(OCCCCCOC(CC[N@+]1(C)[C@H](CC2=CC=C(OC)C(OC)=C2)C3=C(C=C(OC)C(OC)=C3)CC1)=O)CC[N@+]4(C)[C@H](CC5=CC=C(OC)C(OC)=C5)C6=C(C=C(OC)C(OC)=C6)CC4.O=S(C7=CC=CC=C7)([O-])=O.O=S(C8=CC=CC=C8)([O-])=O
Clinical Information
– Launched
Research Area
– Neurological Disease; Cancer
Solubility
– H2O : ≥ 50 mg/mL
Target
– Autophagy;nAChR
Pathway
– Autophagy;Membrane Transporter/Ion Channel;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






