Download Files:
Cetirizine Impurity C (dihydrochloride)
SKU
HY-131256A-10 mg
Category Reference compound
Tags Endocrinology; Inflammation/Immunology, GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling, Histamine Receptor
$300 – $480
Products Details
Product Description
– Cetirizine Impurity C dihydrochloride is an impurity of Cetirizine. Cetirizine, a second-generation antihistamine and the carboxylated metabolite of hydroxyzine, is a specific, orally active and long-acting histamine H1-receptor antagonist[1][2].
Web ID
– HY-131256A
Storage Temperature
– -20°C (Powder, sealed storage, away from moisture)
Shipping
– Blue Ice
Applications
– Metabolism-protein/nucleotide metabolism
Molecular Formula
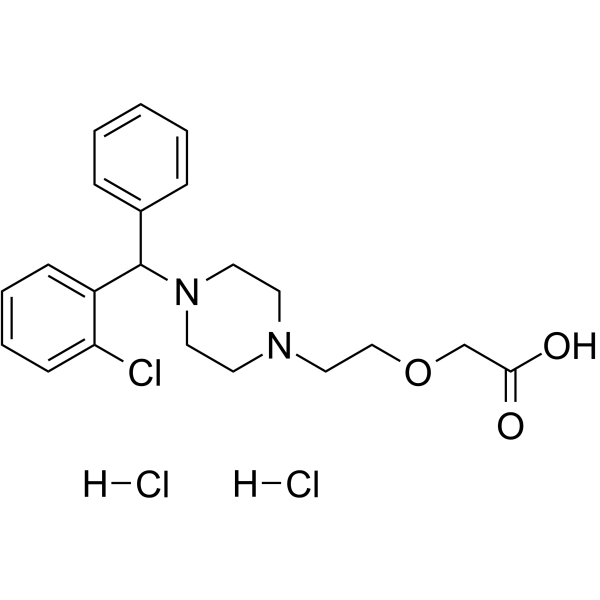
– C21H27Cl3N2O3
References
– [1]Shih MY, et al. Influence of cetirizine and levocetirizine on two cytokines secretion in human airway epithelial cells. Allergy Asthma Proc. 2008 Sep-Oct;29(5):480-5.|[2]Shimizu T, et al. Cetirizine, an H1-receptor antagonist, suppresses the expression of macrophage migration inhibitory factor: its potential anti-inflammatory action. Clin Exp Allergy. 2004 Jan;34(1):103-9.
CAS Number
– 2702511-37-1
Molecular Weight
– 461.81
Compound Purity
– 99.95
SMILES
– O=C(O)COCCN1CCN(C(C2=CC=CC=C2Cl)C3=CC=CC=C3)CC1.[H]Cl.[H]Cl
Clinical Information
– No Development Reported
Research Area
– Endocrinology; Inflammation/Immunology
Solubility
– DMSO : 100 mg/mL (ultrasonic)
Target
– Histamine Receptor
Isoform
– H1 Receptor
Pathway
– GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.



![9-β-D-[2'-Fluoro-2'-deoxy-arabinofuranosyl]-guanin](https://immunomart.com/wp-content/uploads/2024/02/HY-W334680-350x350.gif)



