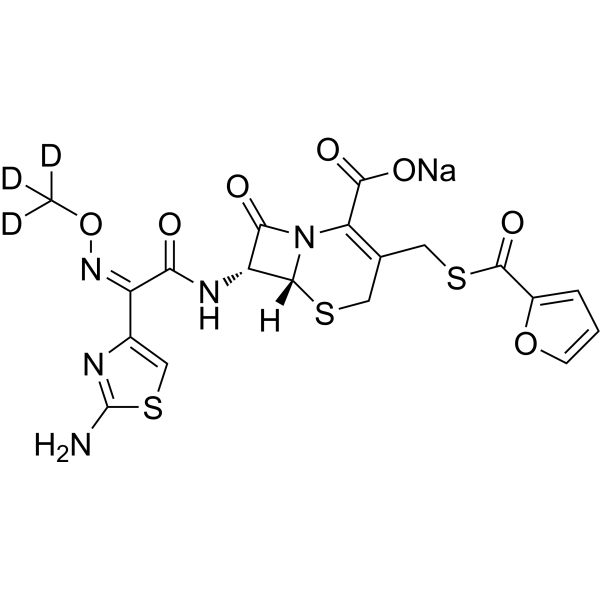
Download Files:
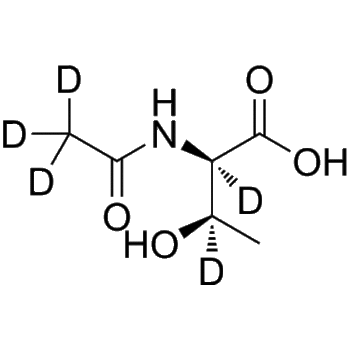
Ceftiofur-d3 (sodium)
SKU
HY-B0898S-1mg
Category Isotope-Labeled Compounds
Tags Anti-infection;Others, Antibiotic;Bacterial;Isotope-Labeled Compounds, Infection
Products Details
Product Description
– Ceftiofur-d3 (sodium) is deuterium labeled Ceftiofur (sodium).
Web ID
– HY-B0898S
Shipping
– Room temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
– C19H13D3N5NaO7S3
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216.|[2]Dutil L, et al. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerg Infect Dis. 2010 Jan;16(1):48-54.|[3]Salmon SA, et al. In vitro activity of ceftiofur and its primary metabolite, desfuroylceftiofur, against organisms of veterinary importance. J Vet Diagn Invest. 1996 Jul;8(3):332-6.|[4]Yancey RJ Jr, et al. Ceftiofur sodium, a broad-spectrum cephalosporin: evaluation in vitro and in vivo in mice. Am J Vet Res. 1987 Jul;48(7):1050-3.
Molecular Weight
– 548.56
SMILES
– O=C(C(N12)=C(CSC(C3=CC=CO3)=O)CS[C@]2([H])[C@H](NC(/C(C4=CSC(N)=N4)=NOC([2H])([2H])[2H])=O)C1=O)O[Na]
Clinical Information
– No Development Reported
Research Area
– Infection
Solubility
– 10 mM in DMSO
Target
– Antibiotic;Bacterial;Isotope-Labeled Compounds
Isoform
– β-lactam
Pathway
– Anti-infection;Others
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.