Download Files:
Brigatinib
SKU
HY-12857-10 mg
Category Reference compound
Tags Anaplastic lymphoma kinase (ALK), Cancer, Protein Tyrosine Kinase/RTK
$55 – $209
Products Details
Product Description
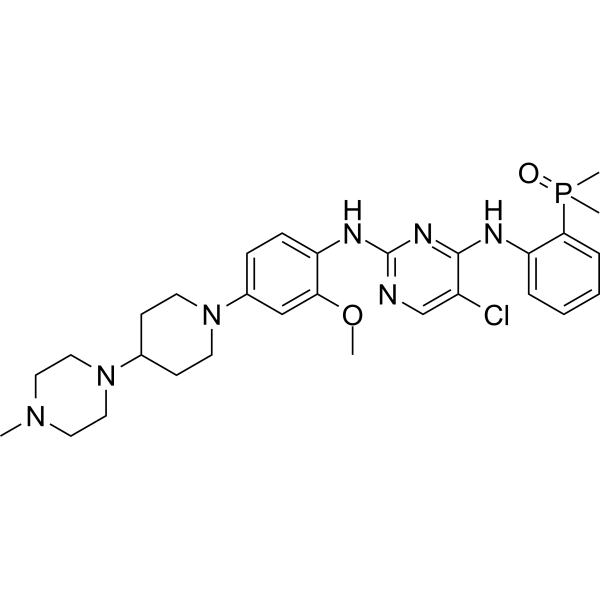
– Brigatinib (AP-26113) is a highly potent and selective ALK inhibitor, with an IC50 of 0.6 nM[1].
Web ID
– HY-12857
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C29H39ClN7O2P
References
– [1]Siaw JT, et al. Brigatinib, an anaplastic lymphoma kinase inhibitor, abrogates activity and growth in ALK-positive neuroblastoma cells, Drosophila and mice. Oncotarget. 2016 May 17;7(20):29011-22|[2]Huang WS, et al. Discovery of Brigatinib (AP26113), a Phosphine Oxide-Containing, Potent, Orally Active Inhibitor of Anaplastic Lymphoma Kinase. J Med Chem. 2016 May 26;59(10):4948-64.|[3]Zhang S, et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin Cancer Res. 2016 Nov 15;22(22):5527-5538
CAS Number
– 1197953-54-0
Molecular Weight
– 584.09
Compound Purity
– 99.98
SMILES
– CN1CCN(C2CCN(C3=CC=C(NC4=NC=C(Cl)C(NC5=CC=CC=C5P(C)(C)=O)=N4)C(OC)=C3)CC2)CC1
Clinical Information
– Launched
Research Area
– Cancer
Solubility
– DMSO : 2 mg/mL (ultrasonic)|Ethanol : 10 mg/mL (ultrasonic;warming)
Target
– Anaplastic lymphoma kinase (ALK)
Pathway
– Protein Tyrosine Kinase/RTK
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.







