Download Files:
BOLD-100
SKU
HY-16350-10 mg
Category Reference compound
Tags Apoptosis;Cell Cycle/DNA Damage, Apoptosis;DNA/RNA Synthesis, Cancer
$420 – $2,520
Products Details
Product Description
– BOLD-100 (NKP-1339; IT-139) is the first-in-class ruthenium-based anticancer agent in development against solid cancer with limited side effects. BOLD-100 induces G2/M cell cycle arrest, blockage of DNA synthesis, and induction of apoptosis via the mitochondrial pathway. BOLD-100 has a high tumor targeting potential, strongly binds to serum proteins such as albumin and transferrin and activates in the reductive tumor milieu[1].
Web ID
– HY-16350
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
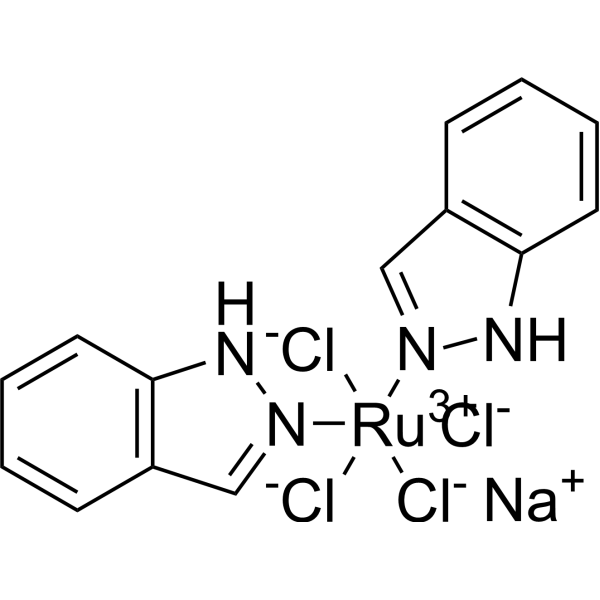
– C14H12Cl4N4NaRu
References
– [1]Robert Trondl, et al. NKP-1339, the first ruthenium-based anticancer drug on the edge to clinical application. Chemical Science. Chemical Science|[2]Heffeter P, et al. The ruthenium compound KP1339 potentiates the anticancer activity of sorafenib in vitro and in vivo. Eur J Cancer. 2013 Oct;49(15):3366-75.
CAS Number
– 197723-00-5
Molecular Weight
– 502.14
Compound Purity
– 98.14
SMILES
– [Cl-][Ru+3]([Cl-])([Cl-])([N]1=CC2=C(N1)C=CC=C2)([N]3=CC4=C(N3)C=CC=C4)[Cl-].[Na+]
Clinical Information
– Phase 2
Research Area
– Cancer
Solubility
– DMSO : ≥ 59 mg/mL
Target
– Apoptosis;DNA/RNA Synthesis
Pathway
– Apoptosis;Cell Cycle/DNA Damage
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.





