Download Files:
Bleomycin
Products Details
Product Description
– Bleomycin is a glycopeptide antibiotic. Bleomycin has potent antitumour activities against a range of lymphomas, head and neck cancers and germ-cell tumours. Bleomycin can be used for the research of cancer and chemotherapy[1][2][3][4].
Web ID
– HY-108345
Shipping
– Room temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– N/A
Citations
– Biomedicines. 2023, 11(2), 463.|BMC Pulm Med. 2023 Nov 13;23(1):440.|Cancer Lett. 2023 Feb 15;558:216092.|Front Biosci (Landmark Ed). 2023 Sep 22, 28(9), 209.|Inflammation. 2023 May 9.|Int Immunopharmacol. 2022, 113: 109409.|Int Immunopharmacol. 2023 May 22;120:110369.|Int J Biol Macromol. 2023 Apr 17;124476.|J Gene Med. 2022 Sep 30;e3451.|MedComm. 2023 Jul 12;4(4):e319.|Mol Med. 2023 Mar 14;29(1):32.|Molecules. 2023, 28(2), 753.|Nat Commun. 2022 Nov 17;13(1):7028.|Phytomedicine. 2023 Jun 17, 154919.|Research Square Preprint. 2023 Aug 3.|Transl Res. 2023 Feb 6;S1931-5244(23)00018-X.|ACS Appl Mater Interfaces. 2019 Jan 16;11(2):1942-1950. |Biochem Biophys Res Commun. 2020 Jun 30;527(3):662-667.|Biochem Pharmacol. 2020 Apr;174:113846.|Biomed Pharmacother. September 2022, 113460.|Cell Death Dis. 2020 Jun 15;11(6):464.|Cell Death Dis. 2020 Nov 12;11(11):976.|Cells. 2021, 10(2), 202.|Cells. 2022 Dec 29;12(1):145.|Cells. 2023, 12(1), 145.|Chinese Journal of Plastic and Reconstructive Surgery. 23 December 2021.|Front Pharmacol. 2022 May 20;13:911945.|Fundamental Research. 23 December 2021.|Gene Rep. 2023 Jul 22, 101823.|Int Immunopharmacol. 2023 Jan 23;116:109723.|Int Immunopharmacol. 2023 Sep 15;124(Pt A):110905.|Mutat Res Genet Toxicol Environ Mutagen. 2016 Sep 15;808:27-37. |OncoImmunology. 2020 Sep 22;9(1):1824631.|Patent. US20190255042A1.|Patent. US20190282565A1.|Phytomedicine. 2023 Feb 11.|Research Square Preprint. 2023 Aug 30.|Research Square Preprint. 2023 Nov 23.|Research Square Print. November 28th, 2022.|Respir Res. 2020 Nov 2;21(1):290.|Respir Res. 2020 Feb 19;21(1):58. |Toxicol Lett. 2022 Apr 23;363:45-54.|Universidad de las Illes Balears. Facultad de Ciencias, 2022 Feb.
References
– [1]Olive PL, et al. Detection of DNA double-strand breaks through the cell cycle after exposure to X-rays, bleomycin, etoposide and 125IdUrd. Int J Radiat Biol. 1993 Oct;64(4):349-58. |[2]Allio T, Preston RJ. Increased sensitivity to chromatid aberration induction by bleomycin and neocarzinostatin results from alterations in a DNA damage response pathway. Mutat Res. 2000 Sep 20;453(1):5-15.|[3]Mekid H, et al. In vivo evolution of tumour cells after the generation of double-strand DNA breaks. Br J Cancer. 2003 Jun 2;88(11):1763-71. |[4]Chen J, Stubbe J. Bleomycins: towards better therapeutics. Nat Rev Cancer. 2005;5(2):102-112.
CAS Number
– 11056-06-7
Molecular Weight
– N/A
SMILES
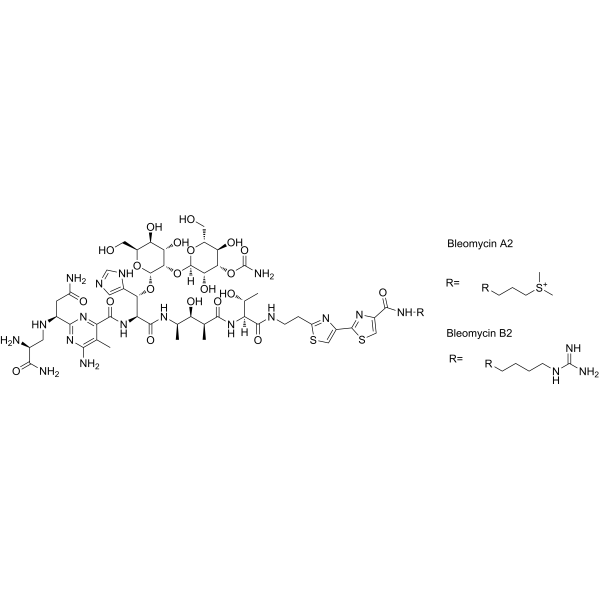
– O=C(C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C)N[C@H](C(N[C@H](C)[C@@H](O)[C@H](C)C(N[C@]([C@H](O)C)([H])C(NCCC2=NC(C3=NC(C(N[R])=O)=CS3)=CS2)=O)=O)=O)[C@H](C4=CN=CN4)O[C@H]5[C@H]([C@H]([C@@H]([C@@H](O5)CO)O)O)O[C@]6([H])[C@H]([C@H]([C@@H]([C@H](O6)CO)O)OC(N)=O)O.NC(NCCCC[R])=N.[R]CCC[S+](C)C.[R=].[Bleomycin A2].[R=].[Bleomycin B2]
Clinical Information
– Launched
Research Area
– Cancer
Solubility
– 10 mM in DMSO
Target
– Antibiotic
Isoform
– Glycopeptide
Pathway
– Anti-infection
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.





