Download Files:
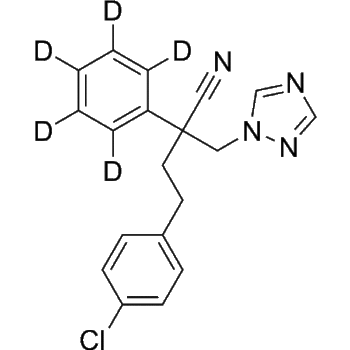
Bilastine-d6
$570
Only 1000 item(s) left in stock.
Products Details
Product Description
– Bilastine-d6 is the deuterium labeled Bilastine. Bilastine is a selective histamine H1 receptor antagonist used for treatment of allergic rhinoconjunctivitis and urticaria[1][2].
Web ID
– HY-14447S
Shipping
– Room temperature
Molecular Formula
– C28H31D6N3O3
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216. |[2]Corcostegui, R., et al., Preclinical pharmacology of bilastine, a new selective histamine H1 receptor antagonist: receptor selectivity and in vitro antihistaminic activity. Drugs R D, 2005. 6(6): p. 371-84.;Jauregizar, N., et al., Pharmacokinetic-pharmacodynamic modelling of the antihistaminic (H1) effect of bilastine. Clin Pharmacokinet, 2009. 48(8): p. 543-54.
CAS Number
– 1215358-58-9
Molecular Weight
– 469.65
SMILES
– CCOCCN1C(C2CCN(CC2)CCC3=CC(C(C([2H])([2H])[2H])(C([2H])([2H])[2H])C(O)=O)=CC=C3)=NC4=CC=CC=C41
Clinical Information
– No Development Reported
Research Area
– Inflammation/Immunology; Endocrinology
Solubility
– 10 mM in DMSO
Target
– Histamine Receptor;Isotope-Labeled Compounds
Isoform
– H1 Receptor
Pathway
– GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling;Others
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.