Download Files:
Bemfivastatin (hemicalcium)
SKU
HY-106281A-10 mg
Category Reference compound
Tags HMG-CoA Reductase (HMGCR), Metabolic Disease; Cardiovascular Disease, Metabolic Enzyme/Protease
$150 – $1,250
Products Details
Product Description
– Bemfivastatin (PPD 10558) hemicalcium is an orally active lipid-lowering agent and HMG-CoA reductase inhibitor. Bemfivastatin hemicalcium enhances the activity of liver extracts. Bemfivastatin hemicalcium has no-observed adverse effect levels (NOAEL) with dosages of ≥320 mg/kg/d (rat developmental toxicity), ≥12.5 mg/kg/d (rabbit maternal toxicity), ≥25 mg/kg/d (rabbit developmental toxicity), respectively. Bemfivastatin hemicalcium can be used in the study of statin-related hypercholesterolemic myalgia in statin-intolerant patients.
Web ID
– HY-106281A
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture and light)
Shipping
– Room Temperature
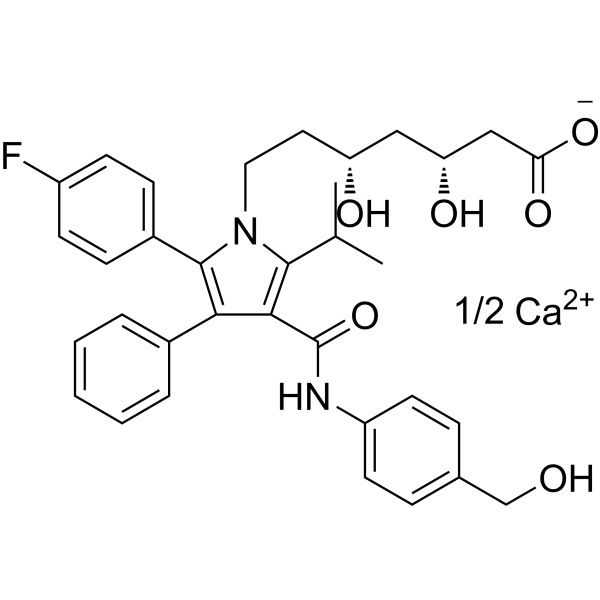
Molecular Formula
– C34H36FN2O6.1/2Ca
References
– [1]Faqi AS, et al. Developmental toxicity of the HMG-CoA reductase inhibitor (PPD10558) in rats and rabbits. Birth Defects Res B Dev Reprod Toxicol. 2012 Feb;95(1):23-37.
CAS Number
– 805241-64-9
Molecular Weight
– 607.74
Compound Purity
– 98.00
SMILES
– [O-]C(C[C@H](O)C[C@H](O)CCN1C(C2=CC=C(F)C=C2)=C(C3=CC=CC=C3)C(C(NC4=CC=C(CO)C=C4)=O)=C1C(C)C)=O.[Ca+2].[1/2]
Clinical Information
– No Development Reported
Research Area
– Metabolic Disease; Cardiovascular Disease
Solubility
– 10 mM in DMSO
Target
– HMG-CoA Reductase (HMGCR)
Pathway
– Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






