Download Files:
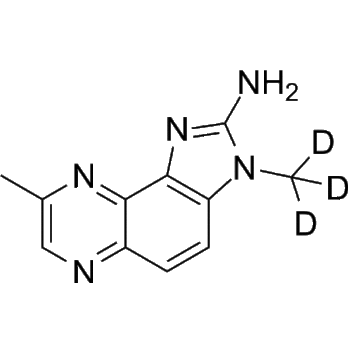
Baricitinib-d3
SKU
HY-15315S1-1mg
Category Isotope-Labeled Compounds
Tags Epigenetics;JAK/STAT Signaling;Protein Tyrosine Kinase/RTK;Stem Cell/Wnt, Inflammation/Immunology, JAK
Products Details
Product Description
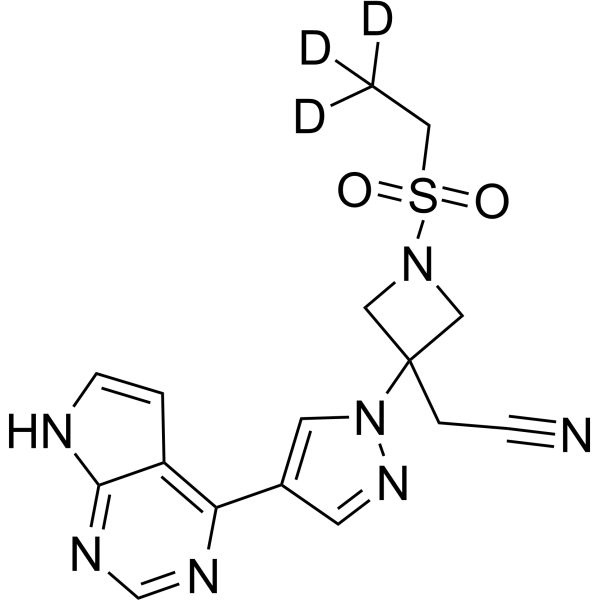
– Baricitinib-d3 is the deuterium labeled Baricitinib. Baricitinib (LY3009104; INCB028050) is a selective and orally bioavailable JAK1 and JAK2 inhibitor with IC50s of 5.9 nM and 5.7 nM, respectively.
Web ID
– HY-15315S1
Shipping
– Room temperature
Molecular Formula
– C16H14D3N7O2S
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216. |[2]Fridman JS, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 2010 May 1;184(9):5298-307.|[3]Jabbari A, et al. Reversal of Alopecia Areata Following Treatment With the JAK1/2 Inhibitor Baricitinib. EBioMedicine. 2015 Feb 26;2(4):351-5.|[4]Khan IM, et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration resistance. Int J Obes (Lond). 2015 Nov;39(11):1607-18.
CAS Number
– 1564242-30-3
Molecular Weight
– 374.44
SMILES
– N#CCC1(N2N=CC(C3=C4C(NC=C4)=NC=N3)=C2)CN(S(=O)(CC([2H])([2H])[2H])=O)C1
Clinical Information
– No Development Reported
Research Area
– Inflammation/Immunology
Solubility
– 10 mM in DMSO
Target
– JAK
Pathway
– Epigenetics;JAK/STAT Signaling;Protein Tyrosine Kinase/RTK;Stem Cell/Wnt
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.