Download Files:
Baloxavir
$209 – $1,440
Products Details
Product Description
– Baloxavir (Baloxavir acid), derived from the proagent Baloxavir marboxil, is a first-in-class, potent and selective cap-dependent endonuclease (CEN) inhibitor within the polymerase PA subunit of influenza A and B viruses. Baloxavir inhibits viral RNA transcription and replication and has potently antiviral activity[1][2].
Web ID
– HY-109025A
Storage Temperature
– 4°C (Powder, stored under nitrogen)
Shipping
– Room Temperature
Applications
– COVID-19-anti-virus
Molecular Formula
– C24H19F2N3O4S
References
– [1]Omoto S, et al. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci Rep. 2018 Jun 25;8(1):9633.|[2]Noshi T, et al. In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antiviral Res. 2018 Dec;160:109-117.
CAS Number
– 1985605-59-1
Molecular Weight
– 483.49
Compound Purity
– 99.80
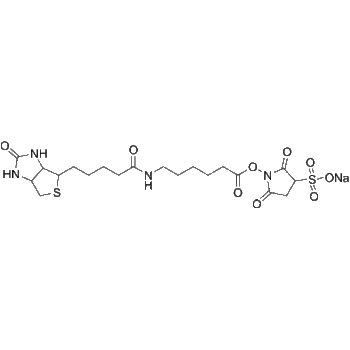
SMILES
– O=C1N2[C@](COCC2)([H])N([C@@H]3C4=CC=CC=C4SCC5=C(F)C(F)=CC=C35)N6C1=C(O)C(C=C6)=O
Clinical Information
– Launched
Research Area
– Infection
Solubility
– DMSO : 41.67 mg/mL (ultrasonic)
Target
– Influenza Virus
Pathway
– Anti-infection
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.