Download Files:
Amisulpride-d5
SKU
HY-14545S-1 mg
Category Isotope-Labeled Compounds
Tags Cancer; Neurological Disease, Dopamine Receptor, GPCR/G Protein;Neuronal Signaling
$240 – $920
Products Details
Product Description
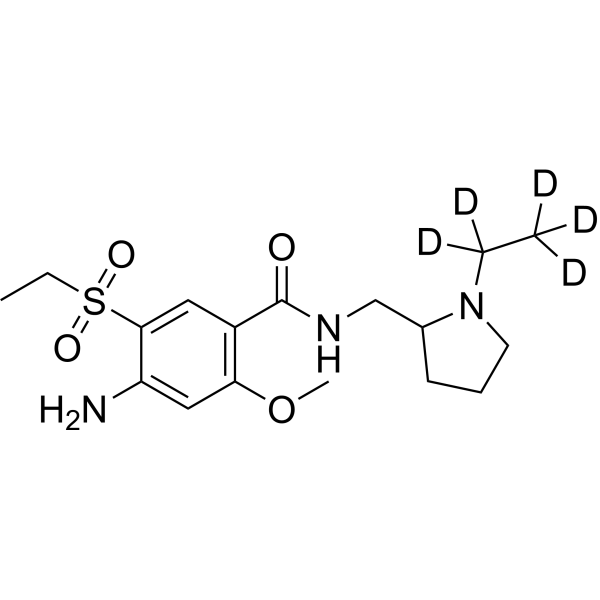
– Amisulpride-d5 is the deuterium labeled Amisulpride. Amisulpride is a dopamine D2/D3 receptor antagonist with Kis of 2.8 and 3.2 nM for human dopamine D2 and D3, respectively[1][2].
Web ID
– HY-14545S
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Molecular Formula
– C17H22D5N3O4S
References
– [1]Pawar GR, et al. Evaluation of antidepressant like property of amisulpride per se and its comparison with fluoxetine and olanzapine using forced swimming test in albino mice. Acta Pol Pharm. 2009 May-Jun;66(3):327-31.|[2]Schoemaker H, et al. Neurochemical characteristics of amisulpride, an atypical dopamine D2/D3 receptor antagonist with both presynaptic and limbic selectivity.
J Pharmacol Exp Ther. 1997 Jan;280(1):83-97.|[3]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216.
CAS Number
– 1216626-17-3
Molecular Weight
– 374.51
Compound Purity
– 98.83
SMILES
– [2H]C([2H])([2H])C([2H])([2H])N(CCC1)C1CNC(C2=C(C=C(C(S(CC)(=O)=O)=C2)N)OC)=O
Clinical Information
– No Development Reported
Research Area
– Cancer; Neurological Disease
Solubility
– 10 mM in DMSO
Target
– Dopamine Receptor
Isoform
– D3 Receptor
Pathway
– GPCR/G Protein;Neuronal Signaling
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.