Download Files:
Alobresib
$85 – $1,800
Products Details
Product Description
– Alobresib (GS-5829) is a BET bromodomain inhibitor, which represents a highly effective therapeutics agent against recurrent/chemotherapy resistant uterine serous carcinoma (USC) overexpressing c-Myc. Alobresib can be used in the metastatic castration-resistant prostate cancer (mCRPC) research[1][2].
Web ID
– HY-109050
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C26H23N5O2
References
– [1]Rahul Aggarwal, et al. Phase Ib Study of the BET Inhibitor GS-5829 as Monotherapy and Combined with Enzalutamide in Patients with Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res. 2022 Sep 15;28(18):3979-3989. |[2]Bonazzoli E, et al. Inhibition of BET Bromodomain Proteins with GS-5829 and GS-626510 in Uterine Serous Carcinoma, a Biologically Aggressive Variant of Endometrial Cancer. Clin Cancer Res. 2018 Oct 1;24(19):4845-4853.
CAS Number
– 1637771-14-2
Molecular Weight
– 437.49
Compound Purity
– 98.04
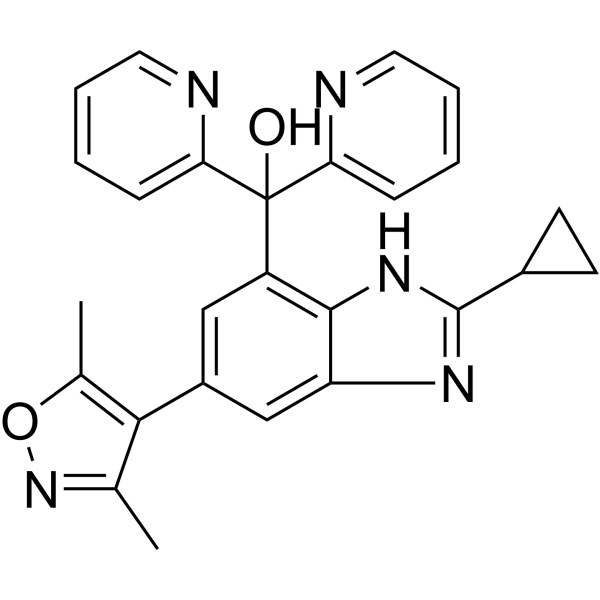
SMILES
– OC(C1=CC=CC=N1)(C2=CC=CC=N2)C3=C(NC(C4CC4)=N5)C5=CC(C(C(C)=NO6)=C6C)=C3
Clinical Information
– Phase 2
Research Area
– Cancer
Solubility
– DMSO : 83.33 mg/mL (ultrasonic)
Target
– Epigenetic Reader Domain
Pathway
– Epigenetics
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






