Download Files:
Almonertinib (mesylate)
SKU
HY-112823A-10 mg
Category Reference compound
Tags Cancer, EGFR, JAK/STAT Signaling;Protein Tyrosine Kinase/RTK
$250 – $1,450
Products Details
Product Description
– Almonertinib (HS-10296) mesylate is an orally available, irreversible, third-generation EGFR tyrosine kinase inhibitor with high selectivity for EGFR-sensitizing and T790M resistance mutations. Almonertinib mesylate shows great inhibitory activity against T790M, T790M/L858R and T790M/Del19 (IC50: 0.37, 0.29 and 0.21 nM, respectively), and is less effective against wild type (3.39 nM). Almonertinib mesylate is used for the research of the non-small cell lung cancer[1][2].
Web ID
– HY-112823A
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
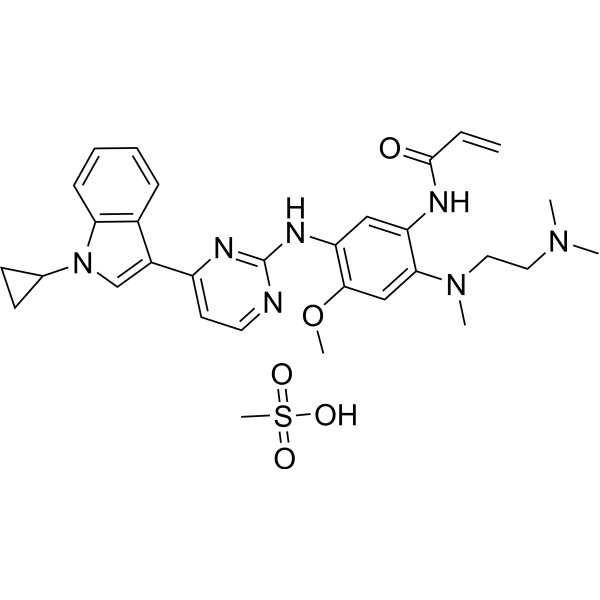
Molecular Formula
– C31H39N7O5S
References
– [1]Sullivan I, et al. Next-Generation EGFR Tyrosine Kinase Inhibitors for Treating EGFR-Mutant Lung Cancer beyond First Line. Front Med (Lausanne). 2017 Jan 18;3:76.|[2]Yang JC, et al. Safety, Efficacy, and Pharmacokinetics of Almonertinib (HS-10296) in Pretreated Patients With EGFR-Mutated Advanced NSCLC: A Multicenter, Open-label, Phase 1 Trial [published online ahead of print, 2020 Sep 9]. J Thorac Oncol. 2020;S1556-0
CAS Number
– 2134096-06-1
Molecular Weight
– 621.75
Compound Purity
– 99.85
SMILES
– COC(C=C(N(C)CCN(C)C)C(NC(C=C)=O)=C1)=C1NC2=NC(C3=CN(C4CC4)C5=C3C=CC=C5)=CC=N2.CS(=O)(O)=O
Clinical Information
– Launched
Research Area
– Cancer
Solubility
– DMSO : 31.25 mg/mL (ultrasonic;warming;heat to 60°C)
Target
– EGFR
Pathway
– JAK/STAT Signaling;Protein Tyrosine Kinase/RTK
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.





