Download Files:
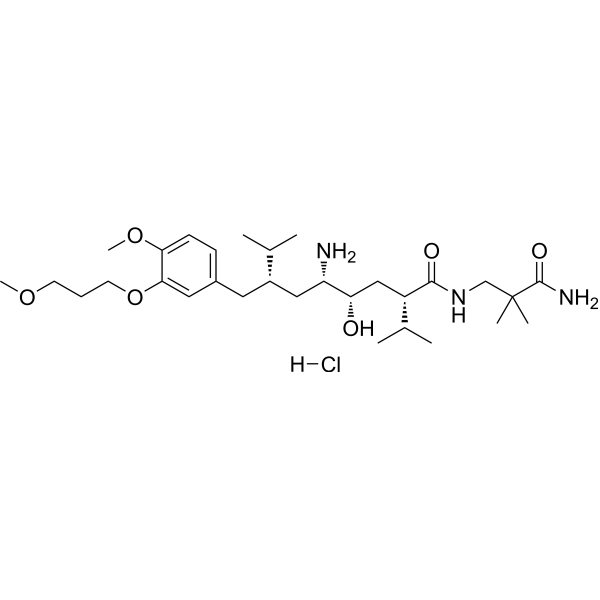
Aliskiren (hydrochloride)
SKU
HY-12176A-Get quote
Category Reference compound
Tags Autophagy;Metabolic Enzyme/Protease, Autophagy;Renin, Cancer; Cardiovascular Disease
Products Details
Product Description
– Aliskiren (CGP 60536; CGP60536B; SPP 100) hydrochloride is an orally active and selective renin inhibitor, with IC50 of 1.5 nM. Aliskiren hydrochloride can be used for the research of hypertension, cardiovascular diseases and cancer cachexia[1]-[4].
Web ID
– HY-12176A
Shipping
– Room temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C30H54ClN3O6
References
– [1]Yuji Nakamura, et al. Discovery of DS-8108b, a Novel Orally Bioavailable Renin Inhibitor. ACS Med. Chem. Lett., 2012, 3 (9), pp 754–758|[2]Wood JM, et al. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun. 2003 Sep 5;308(4):698-705.|[3]Wang C, et al. Aliskiren targets multiple systems to alleviate cancer cachexia. Oncol Rep. 2016 Nov;36(5):3014-3022. |[4]Ferri N, et al. Aliskiren inhibits prorenin-induced human aortic smooth muscle cell migration. J Renin Angiotensin Aldosterone Syst. 2015 Jun;16(2):284-91.
CAS Number
– 173399-03-6
Molecular Weight
– 588.22
SMILES
– [H]Cl.COCCCOC1=CC(C[C@@H](C[C@@H]([C@H](C[C@H](C(NCC(C)(C(N)=O)C)=O)C(C)C)O)N)C(C)C)=CC=C1OC
Clinical Information
– Launched
Research Area
– Cancer; Cardiovascular Disease
Solubility
– 10 mM in DMSO
Target
– Autophagy;Renin
Pathway
– Autophagy;Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.






