Download Files:
Alcaftadine
SKU
HY-17039-10 mg
Category Reference compound
Tags GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling, Histamine Receptor, Inflammation/Immunology; Endocrinology
$42 – $290
Products Details
Product Description
– Alcaftadine (R89674) is a histamine H1 receptor antagonist, which is used to prevent eye irritation brought on by allergic conjunctivitis. Alcaftadine is a broad-spectrum antihistamine displaying a high affinity for histamine H1 and H2 receptors and a lower affinity for H4 receptors. Alcaftadine also exhibits modulatory action on immune cell recruitment and mast cell stabilizing effects[1][2].
Web ID
– HY-17039
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
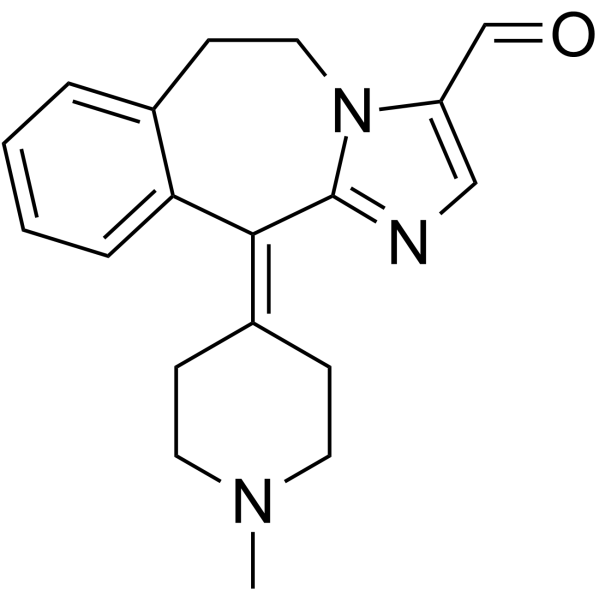
Molecular Formula
– C19H21N3O
References
– [1]Namdar, R. and C. Valdez, Alcaftadine: a topical antihistamine for use in allergic conjunctivitis. Drugs Today (Barc), 2011. 47(12): p. 883-90.|[2]Mahvan, T.D., W.A. Buckley, and J.R. Hornecker, Alcaftadine for the prevention of itching associated with allergic conjunctivitis. Ann Pharmacother, 2012. 46(7-8): p. 1025-32.
CAS Number
– 147084-10-4
Molecular Weight
– 307.39
Compound Purity
– 99.43
SMILES
– O=CC1=CN=C2N1CCC3=CC=CC=C3/C2=C4CCN(C)CC4
Clinical Information
– Launched
Research Area
– Inflammation/Immunology; Endocrinology
Solubility
– DMSO : ≥ 40 mg/mL
Target
– Histamine Receptor
Isoform
– H1 Receptor;H2 Receptor
Pathway
– GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.







