Download Files:
AKT inhibitor VIII
SKU
HY-10355-10 mg
Category Reference compound
Tags Akt;Apoptosis, Apoptosis;PI3K/Akt/mTOR, Cancer; Metabolic Disease
$132 – $858
Products Details
Product Description
– AKT inhibitor VIII (AKTi-1/2) is a cell-permeable quinoxaline compound that has been shown to potently, selectively, allosterically, and reversibly inhibit Akt1, Akt2, and Akt3 activity with IC50s of 58 nM, 210 nM, and 2119 nM, respectively.
Web ID
– HY-10355
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C34H29N7O
References
– [1]Leonardo Romorini, et al. AKT/GSK3β signaling pathway is critically involved in human pluripotent stem cell survival. Sci Rep. 2016; 6: 35660|[2]Zhong Z, et al. Furanodiene, a Natural Product, Inhibits Breast Cancer Growth Both in vitro and in vivo. Cell Physiol Biochem. 2012;30(3):778-90. Epub 2012 Aug 2.|[3]Lindsley, et al. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg. Med. Chem. Lett. (2005), 15(3), 761-764.
CAS Number
– 612847-09-3
Molecular Weight
– 551.64
Compound Purity
– 98.97
SMILES
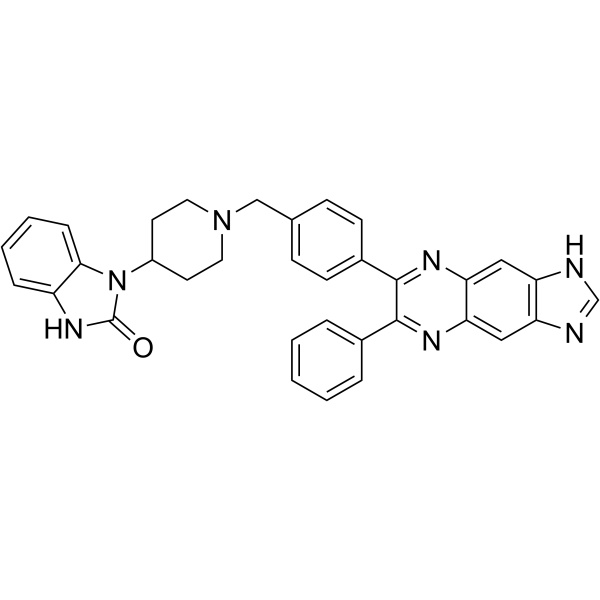
– O=C1NC2=CC=CC=C2N1C3CCN(CC4=CC=C(C5=NC6=CC(NC=N7)=C7C=C6N=C5C8=CC=CC=C8)C=C4)CC3
Clinical Information
– No Development Reported
Research Area
– Cancer; Metabolic Disease
Solubility
– DMSO : 20 mg/mL (ultrasonic)
Target
– Akt;Apoptosis
Isoform
– Akt1;Akt2;Akt3
Pathway
– Apoptosis;PI3K/Akt/mTOR
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.






