Download Files:
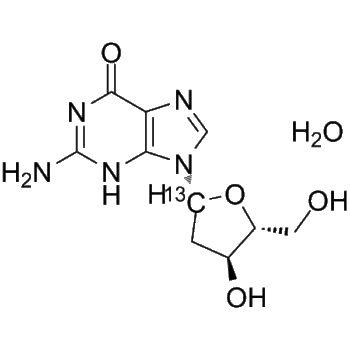
Acyclovir-d4
SKU
HY-17422S1-1 mg
Category Isotope-Labeled Compounds
Tags Anti-infection;Apoptosis;Others, Antibiotic;Apoptosis;Bacterial;HSV;Isotope-Labeled Compounds, Cancer; Infection
$548
Only 1000 item(s) left in stock.
Products Details
Product Description
– Acyclovir-d4 is the deuterium labeled Acyclovir. Acyclovir (Aciclovir) is a guanosine analogue and an orally active antiviral agent. Acyclovir inhibits HSV-1 (IC50 of 0.85 μM), HSV-2 (IC50 of 0.86 μM) and varicella-zoster virus. Acyclovir can be phosphorylated by viral thymidine kinase (TK), and Acyclovir triphosphate interferes with viral DNA polymerization through competitive inhibition with guanosine triphosphate and obligatory chain termination[1][2][3]. Acyclovir prevents bacterial infections during induction therapy for acute leukaemia[4].
Web ID
– HY-17422S1
Shipping
– Room temperature
Molecular Formula
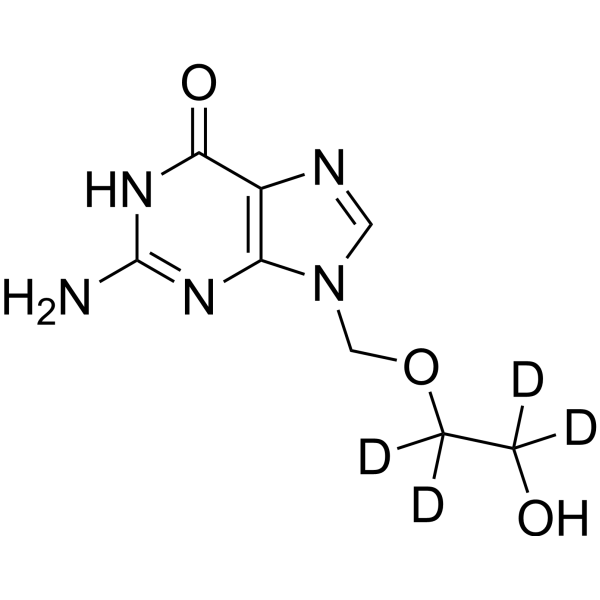
– C8H7D4N5O3
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216. |[2]Li Z, et al. Acyclovir treatment of skin lesions results in immune deviation in mice infected cutaneously with herpes simplex virus. Antivir Chem Chemother. 1999 Sep;10(5):251-7.|[3]Lönnqvist B, et al. Oral acyclovir as prophylaxis for bacterial infections during induction therapy for acute leukaemia in adults. The Leukemia Group of Middle Sweden. Support Care Cancer. 1993 May;1(3):139-44.|[4]Suzuki M, et al. Synergistic antiviral activity of acyclovir and vidarabine against herpes simplex virus types 1 and 2 and varicella-zoster virus. Antiviral Res. 2006 Nov;72(2):157-61.|[5]Benedetti S, et al. Acyclovir induces cell cycle perturbation and apoptosis in Jurkat leukemia cells, and enhances chemotherapeutic drug cytotoxicity. Life Sci. 2018 Dec 15;215:80-85.|[6]Hayashi K, et al. The role of a HSV thymidine kinase stimulating substance, scopadulciol, in improving the efficacy of cancer gene therapy. J Gene Med. 2006 Aug;8(8):1056-67.
CAS Number
– 1185179-33-2
Molecular Weight
– 229.23
SMILES
– O=C1NC(N)=NC2=C1N=CN2COC([2H])([2H])C([2H])([2H])O
Clinical Information
– No Development Reported
Research Area
– Cancer; Infection
Solubility
– 10 mM in DMSO
Target
– Antibiotic;Apoptosis;Bacterial;HSV;Isotope-Labeled Compounds
Pathway
– Anti-infection;Apoptosis;Others
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.