Download Files:
PF-02413873
SKU
HY-11028-10 mg
Category Reference compound
Tags Endocrinology, Progesterone Receptor, Vitamin D Related/Nuclear Receptor
$100 – $820
Products Details
Product Description
– PF-02413873 (PF-2413873) is a potent selective, fully competitive and orally active nonsteroidal progesterone receptor (PR) antagonist, with a Ki of 2.6 nM. PF-02413873 can block progesterone binding and PR nuclear translocation, and inhibit endometrial growth in vivo[1][2].
Web ID
– HY-11028
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Metabolism-protein/nucleotide metabolism
Molecular Formula
– C18H21N3O3S
References
– [1]Howe DC, et, al. The translational efficacy of a nonsteroidal progesterone receptor antagonist, 4-[3-cyclopropyl-1-(mesylmethyl)-5-methyl-1H-pyrazol-4-yl]oxy,-2,6-dimethylbenzonitrile (PF-02413873), on endometrial growth in macaque and human. J Pharmacol Exp Ther. 2011 Nov;339(2):642-53.|[2]Bungay PJ, et, al. Preclinical and clinical pharmacokinetics of PF-02413873, a nonsteroidal progesterone receptor antagonist. Drug Metab Dispos. 2011 Aug;39(8):1396-405.
CAS Number
– 936345-35-6
Molecular Weight
– 359.44
Compound Purity
– 99.29
SMILES
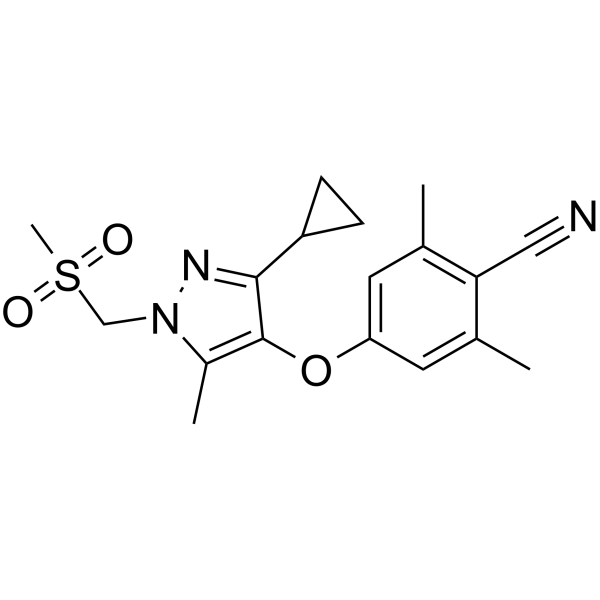
– N#CC1=C(C)C=C(OC2=C(C)N(CS(=O)(C)=O)N=C2C3CC3)C=C1C
Clinical Information
– Phase 1
Research Area
– Endocrinology
Solubility
– DMSO : 100 mg/mL (ultrasonic;warming;heat to 60°C)
Target
– Progesterone Receptor
Pathway
– Vitamin D Related/Nuclear Receptor
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.






