Download Files:
Paltusotine
SKU
HY-109155-10 mg
Category Reference compound
Tags Endocrinology; Cancer, GPCR/G Protein;Neuronal Signaling, Somatostatin Receptor
$650 – $4,830
Products Details
Product Description
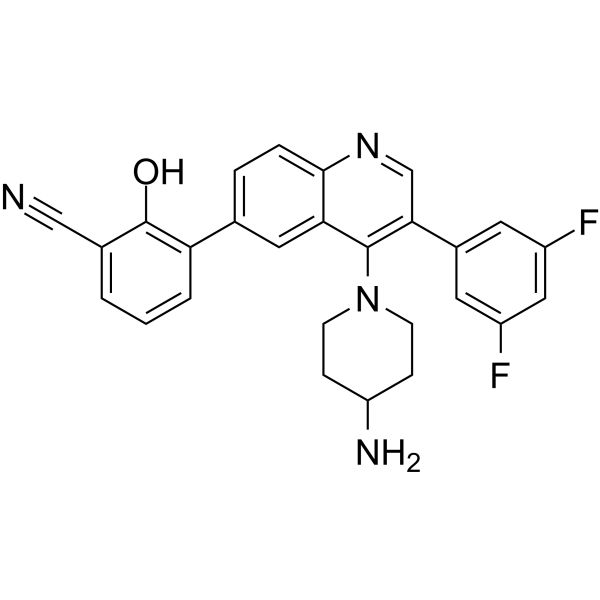
– Paltusotine (CRN00808) is an orally active, nonpeptide selective somatostatin type 2 (SST2) receptor agonist. Paltusotine has the potential for maintaining GH and IGF-1 levels after depot somatostatin receptor ligand therapy[1][2].
Web ID
– HY-109155
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Metabolism-protein/nucleotide metabolism
Molecular Formula
– C27H22F2N4O
References
– [1]Murray B. Gordon, et al. Identification of a dose range for once daily oral paltusotine in patients with acromegaly that maintains IGF-1 levels when switching from long-acting somatostatin receptor ligand therapy. Endocrine Abstracts (2021) 73 OC15.4.|[2]Rosa Luo, et al. Pharmacokinetics and Safety of an Improved Oral Formulation of Paltusotine, a Selective, Non-Peptide Somatostatin Receptor 2 (SST2) Agonist for the Treatment of Acromegaly. Journal of the Endocrine Society, Volume 5, Issue Supplement_1, April-May 2021.
CAS Number
– 2172870-89-0
Molecular Weight
– 456.49
Compound Purity
– 99.06
SMILES
– N#CC1=CC=CC(C2=CC=C3N=CC(C4=CC(F)=CC(F)=C4)=C(N5CCC(N)CC5)C3=C2)=C1O
Clinical Information
– Phase 3
Research Area
– Endocrinology; Cancer
Solubility
– DMSO : 20 mg/mL (ultrasonic;warming;heat to 60°C)
Target
– Somatostatin Receptor
Pathway
– GPCR/G Protein;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.





