Download Files:
ABT-072
$250 – $2,800
Products Details
Product Description
– ABT-072 is an orally active and potent non-nucleoside HCV NS5B polymerase inhibitor (HCV GT1a EC50=1 nM; HCV GT1b EC50=0.3 nM)[1][2][3].
Web ID
– HY-101634
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-anti-virus
Molecular Formula
– C24H27N3O5S
References
– [1]Lawitz E, et al. A phase 2a trial of 12-week interferon-free therapy with two direct-acting antivirals (ABT-450/r, ABT-072) and ribavirin in IL28B C/C patients with chronic hepatitis C genotype 1. J Hepatol. 2013;59(1):18-23.|[2]Shi Y, et al. Assessing Supersaturation and Its Impact on In Vivo Bioavailability of a Low-Solubility Compound ABT-072 With a Dual pH, Two-Phase Dissolution Method. J Pharm Sci. 2016;105(9):2886-2895.|[3]Randolph JT, et al. Synthesis and Biological Characterization of Aryl Uracil Inhibitors of Hepatitis C Virus NS5B Polymerase: Discovery of ABT-072, a trans-Stilbene Analog with Good Oral Bioavailability. J Med Chem. 2018;61(3):1153-1163.
CAS Number
– 1132936-00-5
Molecular Weight
– 469.55
Compound Purity
– 99.86
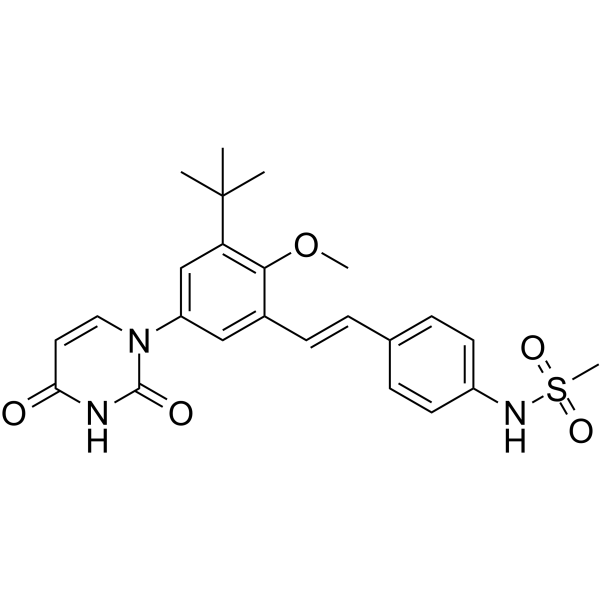
SMILES
– CS(=O)(NC1=CC=C(/C=C/C2=CC(N(C(N3)=O)C=CC3=O)=CC(C(C)(C)C)=C2OC)C=C1)=O
Clinical Information
– Phase 2
Research Area
– Infection
Solubility
– DMSO : 80 mg/mL (ultrasonic)
Target
– HCV
Pathway
– Anti-infection
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.





