Download Files:
Gefapixant
SKU
HY-101588-10 mg
Category Reference compound
Tags Inflammation/Immunology, Membrane Transporter/Ion Channel, P2X Receptor
$110 – $850
Products Details
Product Description
– Gefapixant is an orally active and potent purinergic P2X3 receptor (P2X3R) antagonist, with IC50 values of ~30 nM versus recombinant hP2X3 homotrimers and 100-250 nM at hP2X2/3 heterotrimeric receptors. Gefapixant can be used for the research of chronic cough and knee osteoarthritis[1][2][3].
Web ID
– HY-101588
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-anti-virus
Molecular Formula
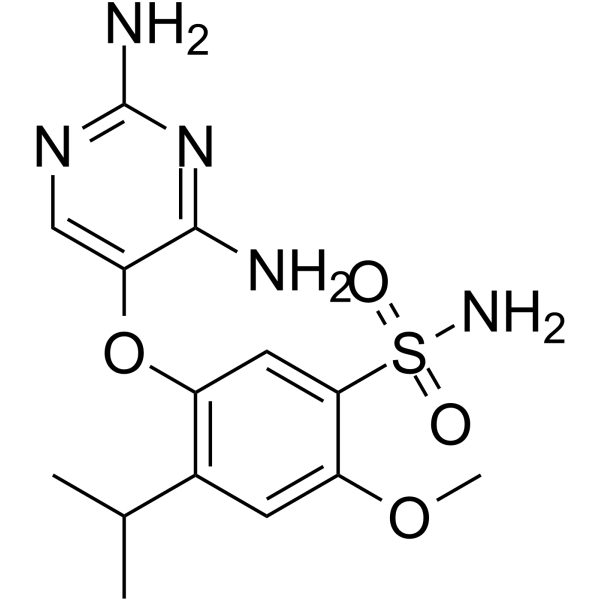
– C14H19N5O4S
References
– [1]Anthony P. Ford, et al. The therapeutic promise of ATP antagonism at P2X3 receptors in respiratory and urological disorders. Front Cell Neurosci. 2013; 7: 267.|[2]Ford AP, In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal. 2012 Feb;8(Suppl 1):3-26.|[3]Martin Nguyen A, et al. Validation of a visual analog scale for assessing cough severity in patients with chronic cough. Ther Adv Respir Dis. 2021 Jan-Dec;15:17534666211049743.
CAS Number
– 1015787-98-0
Molecular Weight
– 353.40
Compound Purity
– 99.32
SMILES
– O=S(C1=CC(OC2=CN=C(N)N=C2N)=C(C(C)C)C=C1OC)(N)=O
Clinical Information
– Launched
Research Area
– Inflammation/Immunology
Solubility
– DMSO : 5 mg/mL (ultrasonic;adjust pH to 5-6 with HCl)
Target
– P2X Receptor
Isoform
– P2X3 Receptor
Pathway
– Membrane Transporter/Ion Channel
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.





