Download Files:
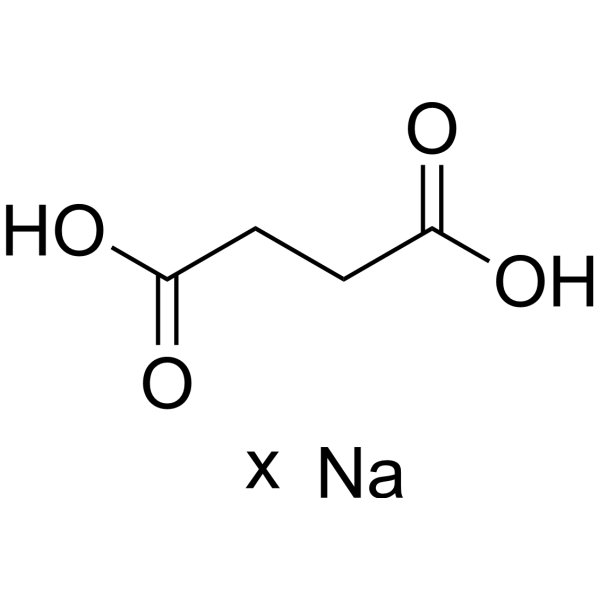
Succinic acid (sodium)
SKU
HY-W396714-Get quote
Category Reference compound
Tags Epigenetics, Membrane Transporter/Ion Channel, Neurological Disease, Potassium Channel, TET Protein
Size – Get quote
Products Details
Product Description
– Succinic acid sodium is a potent and orally active anxiolytic agent. Succinic acid sodium shows inhibitory effects on colonic epithelial cell proliferation in vivo. Succinic acid sodium can down-regulate the expression of KCNMB1 (potassium channel subunit β1) and TET1 (ten‑eleven translocation 1). Succinic acid sodium can be used for gestational hypertension research[1][2][3].
Web ID
– HY-W396714
Shipping
– Room temperature
Applications
– Neuroscience-Neuromodulation
Molecular Formula
– C4H6O4.xNa
References
– [1]Chen SW, Xin Q, Kong WX, Min L, Li JF. Anxiolytic-like effect of succinic acid in mice. Life Sci. 2003 Nov 7;73(25):3257-64. |[2]Inagaki A, et al. Inhibitory effect of succinic acid on epithelial cell proliferation of colonic mucosa in rats. J Nutr Sci Vitaminol (Tokyo). 2007 Aug;53(4):377-9. |[3]Zhou Y, et al. Fumaric acid and succinic acid treat gestational hypertension by downregulating the expression of KCNMB1 and TET1. Exp Ther Med. 2021 Oct;22(4):1072.
CAS Number
– 14047-56-4
Molecular Weight
– N/A
SMILES
– O=C(CCC(O)=O)O.[Na].[x]
Clinical Information
– Launched
Research Area
– Neurological Disease
Solubility
– 10 mM in DMSO
Target
– Potassium Channel, TET Protein
Isoform
– TET1
Pathway
– Epigenetics, Membrane Transporter/Ion Channel
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.





