Download Files:
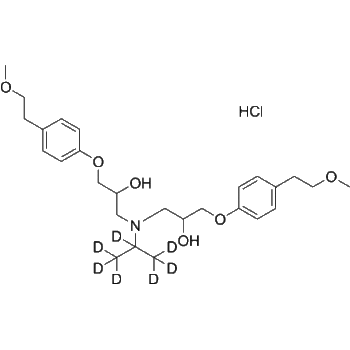
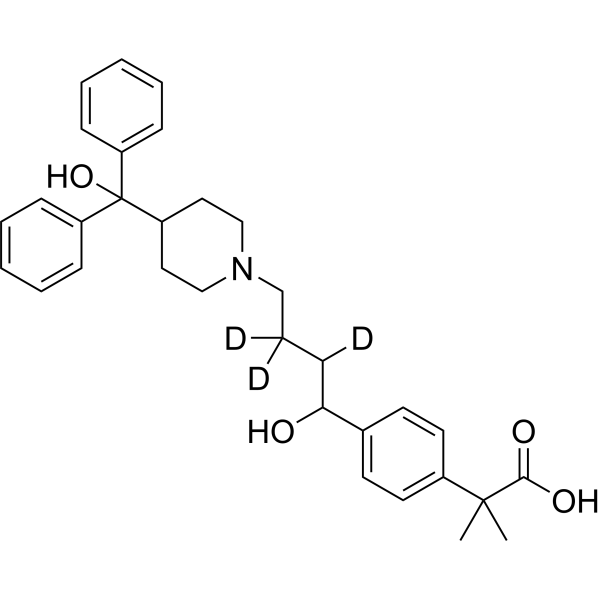
Fexofenadine-d3
SKU
HY-B0801S2-Get quote
Category Isotope-Labeled Compounds
Tags GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling, Histamine Receptor, Inflammation/Immunology
Products Details
Product Description
– Fexofenadine-d3 is the deuterium labeled Fexofenadine[1]. Fexofenadine (MDL-16455) is an orally active and nonsedative H1 receptor antagonist. Fexofenadine can be used in allergic rhinitis and chronic idiopathic urticarial research[2][3][4].
Web ID
– HY-B0801S2
Shipping
– Room temperature
Molecular Formula
– C32H36D3NO4
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019 Feb;53(2):211-216.|[2]Watanabe N, et al. The effects of fexofenadine on eosinophilia and systemic anaphylaxis in mice infected with Trichinella spiralis. Int Immunopharmacol. 2004 Mar;4(3):367-75.|[3]Park IH, et al. Histamine Promotes the Release of Interleukin-6 via the H1R/p38 and NF-κB Pathways in Nasal Fibroblasts. Allergy Asthma Immunol Res. 2014 Nov;6(6):567-72.|[4]Ming X, et al. Vectorial transport of fexofenadine across Caco-2 cells: involvement of apical uptake and basolateral efflux transporters. Mol Pharm. 2011 Oct 3;8(5):1677-86.
Molecular Weight
– 504.67
SMILES
– OC(C1=CC=CC=C1)(C2=CC=CC=C2)C3CCN(CC([2H])([2H])C([2H])C(C4=CC=C(C(C(O)=O)(C)C)C=C4)O)CC3
Clinical Information
– No Development Reported
Research Area
– Inflammation/Immunology
Solubility
– 10 mM in DMSO
Target
– Histamine Receptor
Pathway
– GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.