Download Files:
Fluphenazine (dihydrochloride)
$79
Only 1000 item(s) left in stock.
Products Details
Product Description
– Fluphenazine dihydrochloride is a potent, orally active phenothiazine-based dopamine receptor antagonist. Fluphenazine dihydrochloride blocks neuronal voltage-gated sodium channels. Fluphenazine dihydrochloride acts primarily through antagonism of postsynaptic dopamine-2 receptors in mesolimbic, nigrostriatal, and tuberoinfundibular neural pathways. Fluphenazine dihydrochloride can antagonize Methylphenidate-induced stereotyped gnawing and inhibit climbing behaviour in mice. Fluphenazine dihydrochloride can be used for researching psychosis and painful peripheral neuropathy associated with diabetes and has potential to inhibit SARS-CoV-2[1][2][3][4][6].
Web ID
– HY-A0081
Storage Temperature
– -20°C (Powder, stored under nitrogen)
Shipping
– Blue Ice
Applications
– Cancer-programmed cell death
Molecular Formula
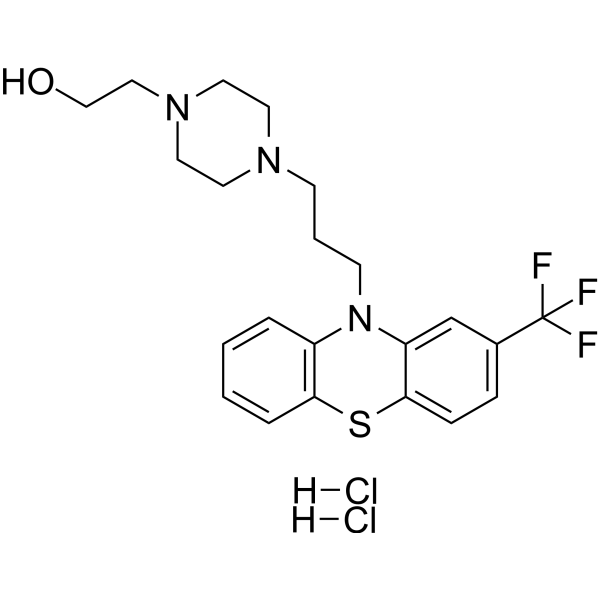
– C22H28Cl2F3N3OS
References
– [1]Davis JL, et al. Peripheral diabetic neuropathy treated with amitriptyline and fluphenazine. JAMA. 1977 Nov 21;238(21):2291-2.|[2]Abdel-Hamid HA, et al. Teratogenic effect of diphenylhydantoin and/or fluphenazine in mice. J Appl Toxicol. 1996 May-Jun;16(3):221-5.|[3]Langwiński R, Niedzielski J. Narcotic analgesics and stereotyped behaviour in mice. Naunyn Schmiedebergs Arch Pharmacol. 1980 Jul;312(3):225-7.|[4]Zhou X, et al. The neuroleptic drug, fluphenazine, blocks neuronal voltage-gated sodium channels. Brain Res. 2006;1106(1):72-81.|[5]Nazeam J, et al. Based on Principles and Insights of COVID-19 Epidemiology, Genome Sequencing, and Pathogenesis: Retrospective Analysis of Sinigrin and ProlixinRX (Fluphenazine) Provides Off-Label Drug Candidates. SLAS Discov. 2020 Dec;25(10):1123-1140.|[6]Siragusa S, Bistas KG, Saadabadi A. Fluphenazine. 2022 May 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan.
CAS Number
– 146-56-5
Molecular Weight
– 510.44
Compound Purity
– 99.84
SMILES
– OCCN1CCN(CCCN2C3=C(C=CC=C3)SC4=CC=C(C(F)(F)F)C=C24)CC1.[H]Cl.[H]Cl
Clinical Information
– Launched
Research Area
– Neurological Disease; Cancer
Solubility
– DMSO : ≥ 38 mg/mL|H2O : 100 mg/mL (ultrasonic)
Target
– Dopamine Receptor;SARS-CoV;Sodium Channel
Pathway
– Anti-infection;GPCR/G Protein;Membrane Transporter/Ion Channel;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.






