Download Files:
Fevipiprant
SKU
HY-16768-10 mg
Category Reference compound
Tags Cardiovascular Disease, CRTH2 (GPR44);Prostaglandin Receptor, GPCR/G Protein;Immunology/Inflammation
$60 – $450
Products Details
Product Description
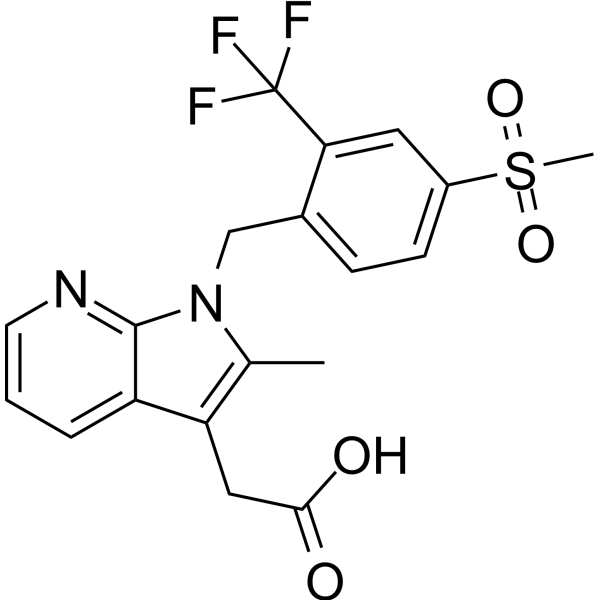
– Fevipiprant (QAW039, NVP-QAW039) is s an orally active, selective, reversible prostaglandin D2 (DP2) receptor antagonist with an Kd value of 1.14 nM. Fevipiprant has the potential for the research of bronchial asthma[1][2][3].
Web ID
– HY-16768
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C19H17F3N2O4S
References
– [1]Brightling C, et al. The pharmacology of the prostaglandin D2 receptor 2 (DP2) receptor antagonist, fevipiprant. Pulm Pharmacol Ther. 2021 Jun;68:102030.|[2]Lee HY, et al. Blockade of thymic stromal lymphopoietin and CRTH2 attenuates airway inflammation in a murine model of allergic asthma. Korean J Intern Med. 2020 May;35(3):619-629. |[3]Weintraub NL, et al. Role of prostaglandin D2 receptors in the pathogenesis of abdominal aortic aneurysm formation. Clin Sci (Lond). 2022 Mar 18;136(5):309-321.
CAS Number
– 872365-14-5
Molecular Weight
– 426.41
Compound Purity
– 99.63
SMILES
– O=C(O)CC1=C(C)N(CC2=CC=C(S(=O)(C)=O)C=C2C(F)(F)F)C3=NC=CC=C31
Clinical Information
– Phase 3
Research Area
– Cardiovascular Disease
Solubility
– DMSO : ≥ 32 mg/mL
Target
– CRTH2 (GPR44);Prostaglandin Receptor
Isoform
– DP
Pathway
– GPCR/G Protein;Immunology/Inflammation
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.