Download Files:
(R)-Merimepodib
$50 – $590
Products Details
Product Description
– (R)-Merimepodib is the inactive isomer of Merimepodib (HY-13986), and can be used as an experimental control. Merimepodib (VX-497) is a noncompetitive and oral inhibitor of inosine monophosphate dehydrogenase (IMPDH) with broad spectrum antiviral activities.
Web ID
– HY-13986A
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Molecular Formula
– C23H24N4O6
References
– [1]Jain J, et al. VX-497: a novel, selective IMPDH inhibitor and immunosuppressive agent. J Pharm Sci. 2001 May;90(5):625-37.|[2]Decker CJ, et al. The novel IMPDH inhibitor VX-497 prolongs skin graft survival and improves graft versus host disease in mice. Drugs Exp Clin Res. 2001;27(3):89-95.|[3]Markland W, et al. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob Agents Chemother. 2000 Apr;44(4):859-66.
Molecular Weight
– 452.46
Compound Purity
– 96.93
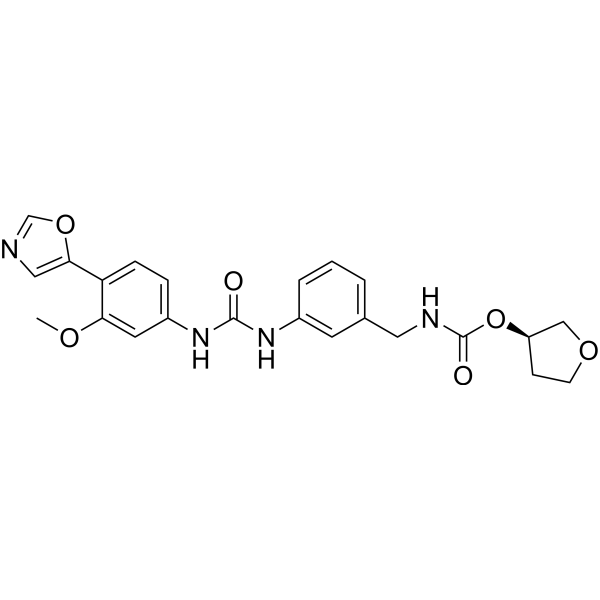
SMILES
– O=C(O[C@H]1COCC1)NCC2=CC=CC(NC(NC3=CC=C(C4=CN=CO4)C(OC)=C3)=O)=C2
Clinical Information
– No Development Reported
Research Area
– Infection
Solubility
– 10 mM in DMSO
Target
– Others
Pathway
– Others
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.






