Download Files:
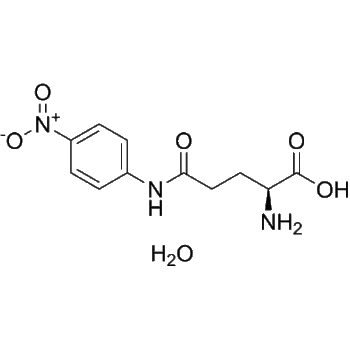
L-Glutamic γ-monohydroxamate
Products Details
Product Description
– L-Glutamic γ-monohydroxamate is an antitumor agent, inhibits cell proliferation. L-Glutamic γ-monohydroxamate selectively inhibits the uptake of L-histidine into microvascular endothelial cell. L-Glutamic γ-monohydroxamate, as a vanadium ligand, activates glucose uptake and metabolism, thus decreases the blood glucose levels in vivo[1][2][3].
Web ID
– HY-137874
Shipping
– Room temperature
Applications
– Metabolism-sugar/lipid metabolism
Molecular Formula
– C5H10N2O4
References
– [1]Vila J, et al. In vitro and in vivo anti-tumor activity of L-glutamic acid gamma-monohydroxamate against L1210 leukemia and B16 melanoma. Int J Cancer. 1990 Apr 15;45(4):737-43.|[2]Sakurai E, et al. Stereoselective transport of histidine in rat lung microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2002 Jun;282(6):L1192-7.|[3]Goldwaser I, et al. L-Glutamic acid gamma-monohydroxamate. A potentiator of vanadium-evoked glucose metabolism in vitro and in vivo. J Biol Chem. 1999 Sep 10;274(37):26617-24.
CAS Number
– 1955-67-5
Molecular Weight
– 162.14
SMILES
– N[C@H](C(O)=O)CCC(NO)=O
Clinical Information
– No Development Reported
Research Area
– Cancer; Metabolic Disease
Solubility
– 10 mM in DMSO
Target
– Others
Pathway
– Others
Product type
– Peptides
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.
Related Products
1000 in stock