Download Files:
Ezatiostat
SKU
HY-13634A-10 mg
Category Peptides
Tags Apoptosis;Gutathione S-transferase, Apoptosis;Metabolic Enzyme/Protease, Cancer
$65 – $580
Products Details
Product Description
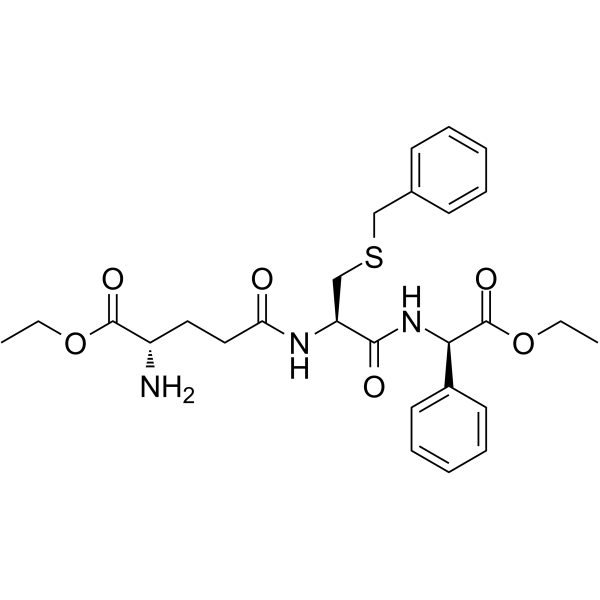
– Ezatiostat (TER199 free base; TLK199) is a tripeptide analog of glutathione and is a selective and orally active glutathione S-transferase P1-1 (GSTP1) inhibitor. Ezatiostat leads to JNK activation by inhibiting GSTP1. Ezatiostat stimulates both lymphocyte production and bone marrow progenitor proliferation. Ezatiostat has the potential for myelodysplastic syndrome (MDS) treatment[1][2].
Web ID
– HY-13634A
Storage Temperature
– -80°C, 2 years; -20°C, 1 year (Powder, sealed storage, away from moisture)
Shipping
– Blue Ice
Applications
– Cancer-Kinase/protease
Molecular Formula
– C27H35N3O6S
References
– [1]Ruscoe JE, et al. Pharmacologic or genetic manipulation of glutathione S-transferase P1-1 (GSTpi) influences cell proliferation pathways. J Pharmacol Exp Ther. 2001 Jul;298(1):339-45.|[2]Galili N, et al. Prediction of response to therapy with ezatiostat in lower risk myelodysplastic syndrome. J Hematol Oncol. 2012 May 6;5:20
CAS Number
– 168682-53-9
Molecular Weight
– 529.65
Compound Purity
– 99.19
SMILES
– O=C(OCC)[C@@H](C1=CC=CC=C1)NC([C@@H](NC(CC[C@H](N)C(OCC)=O)=O)CSCC2=CC=CC=C2)=O
Clinical Information
– Phase 2
Research Area
– Cancer
Solubility
– Ethanol : ≥ 100 mg/mL
Target
– Apoptosis;Gutathione S-transferase
Pathway
– Apoptosis;Metabolic Enzyme/Protease
Product type
– Peptides
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.
Related Products
1000 in stock