Download Files:
Tauro-β-muricholic acid (sodium)
$250
Only 1000 item(s) left in stock.
Products Details
Product Description
– Tauro-β-muricholic Acid sodium (T-βMCA sodium), a endogenous metabolite, is a competitive and reversible farnesoid X receptor (FXR) antagonist, with an IC50 of 40 μM[1][2][3].
Web ID
– HY-135103
Storage Temperature
– -20°C (Powder, stored under nitrogen, away from moisture)
Shipping
– Blue Ice
Applications
– Cancer-programmed cell death
Molecular Formula
– C26H44NNaO7S
References
– [1]Sayin SI, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013 Feb 5;17(2):225-35.|[2]Wahlström A, et al. Induction of farnesoid X receptor signaling in germ-free mice colonized with a human microbiota. J Lipid Res. 2017 Feb;58(2):412-419.|[3]Fu T, et al. FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell. 2019 Feb 21;176(5):1098-1112.e18.
CAS Number
– 145022-92-0
Molecular Weight
– 537.68
Compound Purity
– 98.60
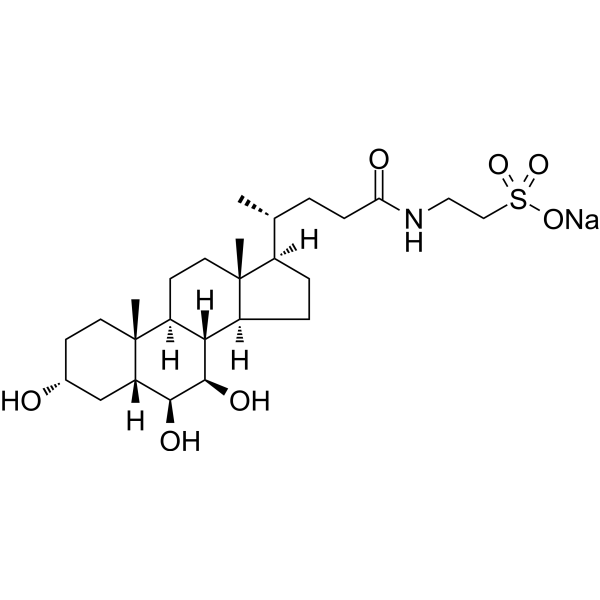
SMILES
– C[C@@]([C@]1([H])[C@H](O)[C@@H]2O)(CC[C@@H](O)C1)[C@]3([H])[C@]2([H])[C@@](CC[C@]4([H])[C@H](C)CCC(NCCS(=O)(O[Na])=O)=O)([H])[C@]4(C)CC3
Clinical Information
– No Development Reported
Research Area
– Cancer
Solubility
– DMSO : 10 mg/mL (ultrasonic;warming)
Target
– FXR
Pathway
– Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.






