Download Files:
Bimiralisib
$66 – $814
Products Details
Product Description
– Bimiralisib (PQR309) is a potent, brain-penetrant, orally bioavailable, pan-class I PI3K/mTOR inhibitor with IC50s of 33 nM, 451 nM, 661 nM, 708 nM and 89 nM for PI3Kα, PI3Kδ, PI3Kβ, PI3Kγ and mTOR, respectively. Bimiralisib is an mTORC1 and mTORC2 inhibitor.
Web ID
– HY-12868
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C17H20F3N7O2
References
– [1]Beaufils F, et al. 5-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine (PQR309), a Potent, Brain-Penetrant, Orally Bioavailable, Pan-Class I PI3K/mTOR Inhibitor as Clinical Candidate in Oncology. J Med Chem. 2017 Sep 14;60(17):7524-7538.|[2]Wicki A, et al. First-in human, phase 1, dose-escalation pharmacokinetic and pharmacodynamic study of the oral dual PI3K and mTORC1/2 inhibitor PQR309 in patients with advanced solid tumors (SAKK 67/13). Eur J Cancer. 2018 Jun;96:6-16.
CAS Number
– 1225037-39-7
Molecular Weight
– 411.38
Compound Purity
– 99.80
SMILES
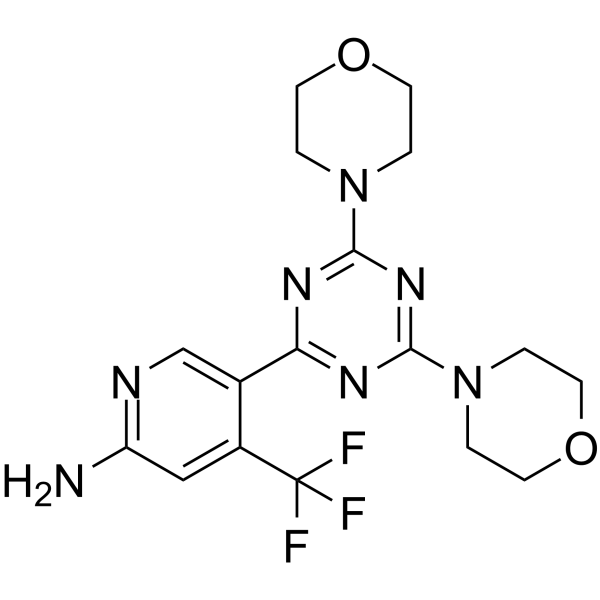
– NC1=NC=C(C2=NC(N3CCOCC3)=NC(N4CCOCC4)=N2)C(C(F)(F)F)=C1
Clinical Information
– Phase 2
Research Area
– Cancer
Solubility
– DMSO : ≥ 50 mg/mL
Target
– mTOR;PI3K
Isoform
– mTOR;mTORC1;mTORC2;PI3Kα;PI3Kβ;PI3Kγ;PI3Kδ;Vps34
Pathway
– PI3K/Akt/mTOR
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.





