Download Files:
Zanzalintinib
SKU
HY-138696-10 mg
Category Reference compound
Tags c-Met/HGFR;TAM Receptor;VEGFR, Cancer, Protein Tyrosine Kinase/RTK
$150 – $1,250
Products Details
Product Description
– Zanzalintinib (XL092) is an orally active, ATP-competitive inhibitor of multiple receptor tyrosine kinases (RTKs) including MET, VEGFR2, AXL and MER, with IC50s in cell-based assays of 15 nM, 1.6 nM, 3.4 nM, 7.2 nM respectively. Zanzalintinib exhibits anti-tumor activity. Zanzalintinib has the potential for kinase-dependent diseases and conditions research[1][2].
Web ID
– HY-138696
Storage Temperature
– 4°C (Powder, protect from light)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C29H25FN4O5
References
– [1]Lynne Canne Bannen, et al. Compounds for the treatment of kinase-dependent disorders. WO2019148044A1.|[2]J. Hsu, et al. XL092, a multi-targeted inhibitor of MET, VEGFR2, AXL and MER with an optimized pharmacokinetic profile. European Journal of Cancer, Volume 138, Supplement 2, October 2020, Page S16.
CAS Number
– 2367004-54-2
Molecular Weight
– 528.53
Compound Purity
– 99.52
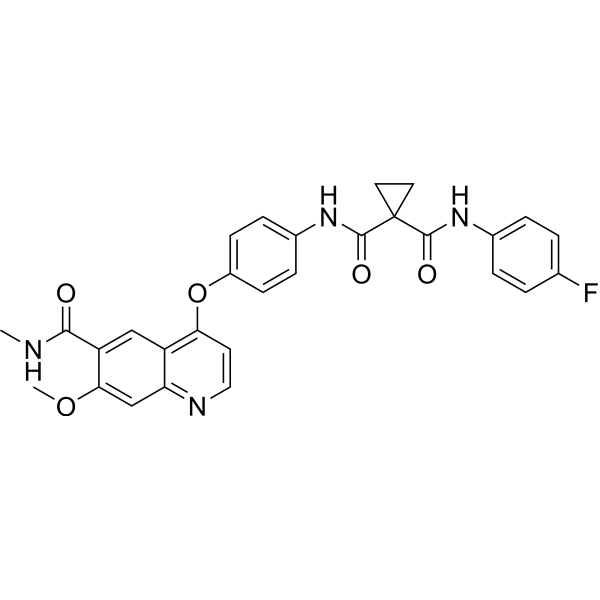
SMILES
– O=C(C1=CC2=C(OC3=CC=C(NC(C4(CC4)C(NC5=CC=C(C=C5)F)=O)=O)C=C3)C=CN=C2C=C1OC)NC
Clinical Information
– Phase 3
Research Area
– Cancer
Solubility
– DMSO : 16.67 mg/mL (ultrasonic;warming;heat to 60°C)
Target
– c-Met/HGFR;TAM Receptor;VEGFR
Isoform
– VEGFR2/KDR/Flk-1
Pathway
– Protein Tyrosine Kinase/RTK
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






