Download Files:
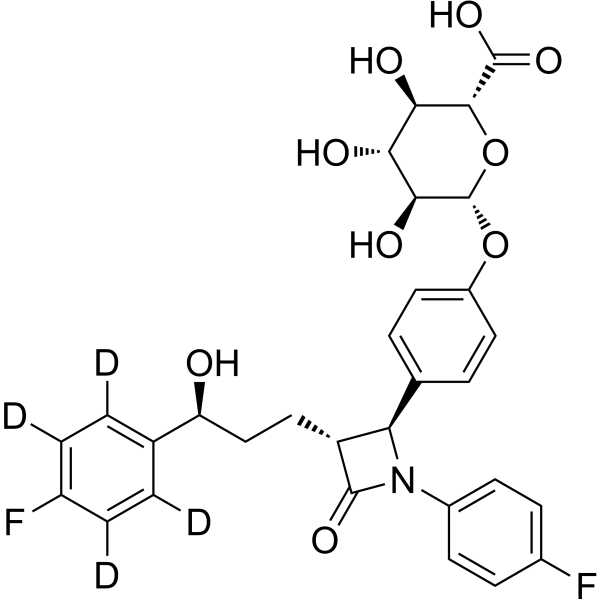
Ezetimibe phenoxy glucuronide-d4-1
SKU
HY-135391S1-Get quote
Category Isotope-Labeled Compounds
Tags Drug Metabolite, Metabolic Enzyme/Protease, Others
Products Details
Product Description
– Ezetimibe phenoxy glucuronide-d4-1 is the deuterium labeled Ezetimibe phenoxy glucuronide. Ezetimibe phenoxy glucuronide (Ezetimibe glucuronide) is the active metabolite of Ezetimibe. Antihyperlipoproteinemic activity[1]. Ezetimibe is a potent cholesterol absorption inhibitor[2][3].
Web ID
– HY-135391S1
Shipping
– Room temperature
Applications
– Metabolism-protein/nucleotide metabolism
Molecular Formula
– C30H25D4F2NO9
References
– [1]Stolk MF, et al. Severe hepatic side effects of ezetimibe. Clin Gastroenterol Hepatol. 2006 Jul;4(7):908-11. |[2]Lee DH, et al. Ezetimibe, an NPC1L1 inhibitor, is a potent Nrf2 activator that protects mice from diet-induced nonalcoholic steatohepatitis. Free Radic Biol Med. 2016 Sep 12;99:520-532. |[3]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-223.
CAS Number
– 2798007-90-4
Molecular Weight
– 589.57
SMILES
– O[C@@H]1[C@@H](OC2=CC=C([C@H]3N(C([C@@H]3CC[C@H](O)C(C([2H])=C(C(F)=C4[2H])[2H])=C4[2H])=O)C5=CC=C(F)C=C5)C=C2)O[C@@H](C(O)=O)[C@H](O)[C@H]1O
Clinical Information
– No Development Reported
Research Area
– Others
Solubility
– 10 mM in DMSO
Target
– Drug Metabolite
Pathway
– Metabolic Enzyme/Protease
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.